Neurogenin2 directs granule neuroblast production and amplification while NeuroD1 specifies neuronal fate during hippocampal neurogenesis - PubMed (original) (raw)
Neurogenin2 directs granule neuroblast production and amplification while NeuroD1 specifies neuronal fate during hippocampal neurogenesis
Laurent Roybon et al. PLoS One. 2009.
Abstract
The specification and differentiation of dentate gyrus granule neurons in the hippocampus require temporally and spatially coordinated actions of both intrinsic and extrinsic molecules. The basic helix-loop-helix transcription factor Neurogenin2 (Ngn2) and NeuroD1 are key regulators in these processes. Based on existing classification, we analyzed the molecular events occurring during hippocampal neurogenesis, primarily focusing on juvenile animals. We found that Ngn2 is transiently expressed by late type-2a amplifying progenitors. The Ngn2 progenies mature into hippocampal granule neurons. Interestingly, the loss of Ngn2 at early stages of development leads to a robust reduction in neurogenesis, but does not disturb granule neuron maturation per se. We found that the role of Ngn2 is to maintain progenitors in an undifferentiated state, allowing them to amplify prior to their maturation into granule neurons upon NeuroD1 induction. When we overexpressed Ngn2 and NeuroD1 in vivo, we found NeuroD1 to exhibit a more pronounced neuron-inductive effect, leading to granule neuron commitment, than that displayed by Ngn2. Finally, we observed that all markers expressed during the transcriptional control of hippocampal neurogenesis in rodents are also present in the human hippocampus. Taken together, we demonstrate a critical role of for Ngn2 and NeuroD1 in controlling neuronal commitment and hippocampal granule neuroblast formation, both during embryonic development and in post-natal hippocampal granule neurogenesis.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
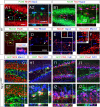
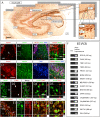
Figure 1. Characterization of the molecular signatures defining each phases of cell maturation during hippocampal granule neuronal differentiation, in two-weeks old newborn mice.
(A1–J2) Indirect immunofluorescence performed on hippocampal coronal sections from 2 weeks old newborn mice. (A1–B2) Type-1 radial glia stem cells are identified by the co-expression of Nestin, Glast, GFAP and Pax6. Radial glia cells locate in both dorsal and ventral blades of the DG. (C1–C2) Type-2 amplifying progenitors expressing Tbr2 rarely co-express PSA-NCAM or Dcx. (D1–D2) Type-3 maturing granule neurons co-express Tbr1, PSA-NCAM and Dcx. (E1–E2) Type-3 maturing granule neurons co-express NeuroD1 but not Pax6. Pax6 progenitors co-expressing NeuroD1 were rarely found and located in the hilus. (F1–F2) Granule neurons maturation occurs through the expression of NeuroD1, PSA-NCAM and Calretinin. (G1–G2) The transition from type-2 amplifying progenitors to mature neurons takes place when the type 2 cells express Tbr2 followed by expression of NeuroD1 and thereafter NeuN. (H1–H2) The transition from type-3 amplifying progenitors to mature granule neurons occurs through the expression of NeuroD1, Tbr1 and NeuN. (I1–I2) The expression of Prox1 starts soon after that of NeuroD1. (J1–J2) NeuroD2 expression starts soon after that of NeuroD1 and persists in mature granule neurons, in contrast to NeuroD1. Panels A2–J2 represent a high magnification of framed areas in corresponding panels A1–J1. Rectangular images on the bottom and right of the panels A2–J2 represent projected images of 14-Z stacks (total of 10–14 µm thick). The white crosshairs in these panels were positioned to show co-expression of markers of interest in single cells. See each panel label. SGZ = subgranular zone, GL = granule layer, DB = dorsal blade and VB = ventral blade. Scale bars: 50 µm (A1, B1, C1, D1, E1, F1, G1, H1, I1 and J1), 25 µm (A2, B2, C2, D2, E2, F2, G2, H2, I2 and J2).
Figure 2. Ngn2+ cells become hippocampal granule neurons.
(A1–J2) Indirect immunofluorescence performed on coronal sections from two-weeks old WT and Ngn2+/GFP mice. (A1–A2) Ngn2-expressing cells co-express Pax6 (>90%), but not NeuN. Ngn2+ cells are often observed by doublet of cells. (B1–B2) Almost all Ngn2+ cells express Tbr2, however only half of Tbr2+ cells co-express Ngn2. (C–F) Mash1 expression precedes that of Ngn2. (C) All Mash1+ cells express Pax6, and exhibit high mitotic activity (See also supplementary Figure 1J). Mash1 expression ceased at the onset of Tbr2 (D) or Ngn2 expression (E). (F) Mash1 cells never co-expressed NeuroD1. (G1–G2) GFP (driven by the Ngn2 promoter) starts upon Mash1 downregulation. Mash1 expression and GFP expression do not co-localize. Ngn2 GFP-expressing progenies become DG granule neurons, the latter expresses NeuroD2 during their maturation. Ngn2 GFP-expressing progenies sequentially express Tbr2 and NeuroD1 (H1–H2), the cellular membrane markers PSA-NCAM and Calretinin (I1–I2) and Prox1, an identity marker of mature hippocampal granule neurons (I1–J2). (K) Expression patterns of cellular markers and transcription factors during hippocampal DG granule neurogenesis. The type-2a stage has been divided into early and late type-2a stages, based on the sequential expression of Mash1 and Ngn2. Panels A2, B2, G2–J2 represent a higher magnification of framed areas in corresponding panels A1, B1, G1–J1. The white crosshairs in these panels were positioned to show co-expression of the markers of interest in single cells. Rectangular images on the bottom and right of the panels A2, B2, D–F and J2–G2 represent a projection of 14-Z stacks images (total of 10–20 µm thick). Scale bars: 50 µm (A1, B1, C, G1, H1, I1 and J1), 25 µm (A2, B2, D, E, F, G2, H2, I2 and J2).
Figure 3. Malformation of the hippocampal DG structure in Ngn2 null mutant animals.
(A1–A3) Hematoxylin/Eosin staining performed on coronal sections from two-weeks old WT, Ngn2+/GFP and Ngn2GFP/GFP mutant animals, shows that the loss of Ngn2 results in the formation of a smaller hippocampus and primitive and undeveloped DG. (B1–B4) The malformation of the DG in Ngn2GFP/GFP mutant animals can already be observed two days after birth, throughout the hippocampal structure, along the rostro-caudal axis (see also supplementary figure 2). The inlets of rectangular panels in bottom right corners show a schematic drawing of the shape of the DG for Ngn2+/GFP and Ngn2GFP/GFP mutant animals; black arrowheads point to the undeveloped and missing dorsal blade and ventral blade of the DG, respectively, in Ngn2GFP/GFP mutant animal. Scale bars: 200 µm (A1–B4).
Figure 4. Ngn2 directs the production of hippocampal DG granule neuroblasts.
(A1–B5, H–I2 and L1–N1) Indirect immunofluorescence performed on hippocampal coronal sections from two-days old Ngn2+/GFP and Ngn2GFP/GFP mutant animals (A1–B5 and H–I2) and two-weeks old Ngn2+/GFP mutant animals (L1–N1). (A1–B5) Presence of BrdU+ cells (red) in the hSVZ, chains of migrating cells and DG of two-days old Ngn2+/GFP and Ngn2GFP/GFP mutant animals injected with BrdU two hours prior to sacrifice. A4, A5, B4 and B5 represent high magnification of the framed areas in A1 and B1, respectively. (C–I2) Cell proliferation and neurogenesis are severely decreased in Ngn2GFP/GFP mutant animals. The histograms C–G and J and K depict the average number or percentage (±SEM); P<0.0001 = *** and P<0.01 = **. (L1–M2) Granule neuron maturation is confirmed by indirect immunofluorescence on hippocampal sections from two-weeks old Ngn2GFP/GFP null mutant animals, as revealed by the co-expression of Calretinin, NeuroD1, Prox1 and NeuN in GFP-expressing cells. (N) In the absence of the Ngn2 protein, GFP-expressing cells do not switch from a neuronal commitment to an astrocytic phenotype. Rectangular images on the bottom and right of the panels L2, M2 and N represent a projection of 14-Z stacks images (total of 10–14 µm thick). The white crosshairs in these panels were positioned to show co-expression of markers of interest in single cells, as labeled above each panel. Scale bars: 200 µm (A1–3 and B1–3), 100 µm (H and H1), 50 µm (A4, A5, B4, B5, L1 and M1), 25 µm (I2, L2, M2 and N).
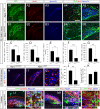
Figure 5. Ngn2 and NeuroD1 mark the initiation and end of amplification of hippocampal DG granule neuroblasts, respectively.
(A1–C3 and D1–K2) Indirect immunofluorescence performed on hippocampal coronal sections from postnatal two-weeks old WT, Ngn2+/GFP and Ngn2GFP/GFP mutant animals and Ngn2 and NeuroD1 retrovirally transduced E14.5 cortico-hippocampal neurospheres. (A1 and A2) Fifty percent of Ngn2+ cells co-label with Ki67. (B1 and B2) One BrdU injection with a two-hour chase labels 50% of the Ngn2-expressing cell population, representing half of the total number of proliferating cells in the DG. (C1–C4) In the DG of two-weeks old Ngn2GFP/GFP mutant animals, the number of dividing cells is substantially decreased compared to WT (data expressed as ±SEM; *** P<0.0001). (D1 and D2) In Ngn2+/GFP mice, GFP/Ngn2-expressing cells undergo division and give rise to GFP+/NeuroD1− cells. These cells have a baso-apical orientation. (E1 and E2) GFP/Ngn2-expressing cells stop dividing when NeuroD1 expression starts. Dividing cells expressing GFP and NeuroD1 have a planar orientation (see also supplementary figure 4). (F1 and G2) All cells brightly GFP present in the ventral blade of the DG of Ngn2GFP/GFP mutant animals co-express Ki67; however, only very few divide, indicating that in absence of Ngn2, Ngn2 progenies stay arrested in the cell cycle. (H1–J) Presence of GFP+/MAP2+/PH3+ dividing neuroblasts in Ngn2-transduced E14.5 hippocampo-cortical neurospheres compared to NeuroD1-transduced cultures. (K–L2) Five days differentiated Ngn2 transduced cells (GFP+) that have been cultures for two more additional days in medium supplemented with CldU, are still capable of dividing after 7 days, as opposed to NeuroD1 transduced cells. Rectangular images on the bottom and right of the panels A2, B2, C3, D2, F2, G2, I1 and J1 represent a projection of 14-Z stacks images (total of 10–14 µm thick) from framed areas or pointing arrows in panels A1, B2, C3, D2, F2, G2, I1 and J1, respectively. The white crosshairs in these panels were positioned to show co-expression in single cells of markers of interest, as labeled above each panel. Arrows and arrowheads point at cells of interest. Scale bars: 100 µm (B1, C1 and C2), 50 µm (A1, D1, E1, F1, G1, H1, I1, K and L1), 25 µm (A2, B2, and F2), 10 µm (L2), 5 µm (C3, E2, G2, H2 and I2).
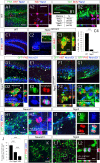
Figure 6. Ngn2 induces NeuroD1 expression, in vitro.
(C1–G2) Indirect immunofluorescence performed on cortico-hippocampal, ventral mesencephalic and lateral ganglionic eminence neurospheres derived from tissue of E14.5 rat embryos. (A) Schematic representation of the retroviral constructs used in this study. (B) Schematic representation of the experimental design. (C1–C4) Upon five days of differentiation, Ngn2- and NeuroD1-transduced cells (GFP+) downregulate the immature neural marker Sox2 (red), as compared to control transduced cells. (D1–D4) Ngn2 and NeuroD1 overexpression induce Tbr1 expression and MAP2. Data in C4 and D4 are expressed as ±SEM; *** P<0.0001 (E1–F) Overexpression of Ngn2 in cortico-hippocampal and ventral mesencephalic neurospheres induces the expression of NeuroD1 and leads to neuronal differentiation, as marked by the expression of PSA-NCAM (red). (G1–G2) The ectopic overexpression of Ngn2 induces ectopic expression of NeuroD1 and neuronal differentiation in LGE neurospheres. (H) Schematic illustration of molecular events upon Ngn2 induction, in vitro. Scale bars: 50 µm (C1–3, D1–3, E1–G2).
Figure 7. NeuroD1 directs neuronal differentiation of hippocampal progenitors in vivo.
(B1–P2) Indirect immunofluorescence performed on hippocampal coronal sections of two-weeks old rats that received a retroviral injection with Ngn2, NeuroD1 or control retroviruses, at the intra-uterine age of E15.5. (A) Schematic representation of the experimental design for in utero retroviral transgene delivery procedure. (B1–B3) Presence of GFP-expressing cells in the hippocampus, three weeks after retroviral injection with Ngn2, NeuroD1 or control vectors. (B4) The proportion of GFP-granule neuron-like cells (blue) and GFP-progenitors/glial-like cells (red) in animals injected with each retrovirus (expressed in percentage of GFP cells) indicate a complete differentiation of NeuroD1-transduced hippocampal progenitors into granule neurons-like cells. (C1–F) Ngn2 overexpression in vivo results in the generation of GFAP-expressing astrocytes, also observed in control group. NeuroD1 overexpression did not induce astrocytic differentiation. (G1–J) In utero injections of control and Ngn2 retroviruses result in the generation of Tbr1+ cells. Only a few Tbr1+/eGFP+ cells were observed in the hippocampal DG of the animals injected with NeuroD1 retrovirus. (K–O) NeuroD1 overexpression induces exclusive neuronal differentiation, in contrast to Ngn2 overexpression. (P1 and P2) A few GFP+ cells that were transduced with Ngn2 retrovirus are found still dividing. Data are expressed as ±SEM; *** P<0.0001 and ** P<0.01. Scale bars: 50 µm (B1–3, C1, D1, E, G1, H1, I, K, L, M1 and P1), 25 µm (C2, D2, G2, H2 and M2), 10 µm (P2).
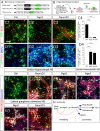
Figure 8. The expression pattern of markers for cell maturation during rodent hippocampal neurogenesis is conserved during human hippocampal neurogenesis.
(A–E5) Indirect immunohistochemistries performed on human hippocampal coronal sections of 58-years-old individual. (A) DAB immunohistochemistry for the neuronal marker NeuN shows the neuro-anatomical structure of the hippocampus. (B1–B4) Mature granule neurons and astrocytes/radial glia-like cells express NeuN (green) and GFAP (red), respectively. (C1–C4) Neurons located in the DG express the granule neuron identity marker Prox1. (D1–D5) Pax6 and NeuN expression do not co-localize, suggesting the existence of astrocytes/radial glial stem cell in human hippocampus. (E1–E5) The presence of Sox2+ cells in the SVZ of the DG suggests the occurrence of ongoing neurogenesis. (F) The existence of radial glia stem cells in the human hippocampus is identified by RT-PCR for the markers Pax6, GFAP and GLAST. The molecular bibliography of cellular markers and transcription factors expressed during hippocampal neurogenesis in rodents is conserved during hippocampal neurogenesis in human. Rectangular images on the bottom and right of the panels B4, D4 and D5, E4 and E5 represent a projection of 14-Z stacks images (total of 10–14 µm thick) from framed areas or pointing arrows in panels B2, D3 and E1. The white crosshairs in these panels were positioned to show co-expression in single cells of markers of interest, as labeled above each panel. Arrows and arrowheads point at cells of interest. CA = cornu ammonis, LV = lateral ventricle, SGZ = sub granular zone, GCL = granule cell layer, SVZ = subventricular zone, h = hilus. Scale bars: 300 µm (A), 50 µm (B1–3, C1–4, D1–3 and E1), 25 µm (B4, D4, D5 and E2–5).
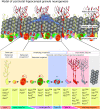
Figure 9. Model of postnatal hippocampal granule neurogenesis.
Multipotent GFAP/Nestin/Pax6/Glast+ radial glia stem cells give rise to multipotent and highly dividing Pax6/Mash1+ progenitors. Ngn2 initiates neuronal commitment of Pax6/Mash1+ progenitors. Ngn2 progenies undergo asymmetric divisions and amplify until they divide symmetrically and express NeuroD1. NeuroD1 stops the amplification phase of Ngn2 progenies and direct neuronal maturation. NeuroD1 progenies undergo maturation through the expression of the transcription factors Tbr1, NeuroD2 and Prox1 and the cellular markers Dcx, PSA-NCAM, Calretinin and NeuN.
Similar articles
- Involvement of Ngn2, Tbr and NeuroD proteins during postnatal olfactory bulb neurogenesis.
Roybon L, Deierborg T, Brundin P, Li JY. Roybon L, et al. Eur J Neurosci. 2009 Jan;29(2):232-43. doi: 10.1111/j.1460-9568.2008.06595.x. Eur J Neurosci. 2009. PMID: 19200230 - Proliferating neuronal progenitors in the postnatal hippocampus transiently express the proneural gene Ngn2.
Ozen I, Galichet C, Watts C, Parras C, Guillemot F, Raineteau O. Ozen I, et al. Eur J Neurosci. 2007 May;25(9):2591-603. doi: 10.1111/j.1460-9568.2007.05541.x. Epub 2007 Apr 27. Eur J Neurosci. 2007. PMID: 17466019 - Genetic manipulation of adult-born hippocampal neurons rescues memory in a mouse model of Alzheimer's disease.
Richetin K, Leclerc C, Toni N, Gallopin T, Pech S, Roybon L, Rampon C. Richetin K, et al. Brain. 2015 Feb;138(Pt 2):440-55. doi: 10.1093/brain/awu354. Epub 2014 Dec 16. Brain. 2015. PMID: 25518958 - Regulation of motor neuron specification by phosphorylation of neurogenin 2.
Ma YC, Song MR, Park JP, Henry Ho HY, Hu L, Kurtev MV, Zieg J, Ma Q, Pfaff SL, Greenberg ME. Ma YC, et al. Neuron. 2008 Apr 10;58(1):65-77. doi: 10.1016/j.neuron.2008.01.037. Neuron. 2008. PMID: 18400164 Free PMC article. Review. - Neurogenesis in the Adult Hippocampus.
Kempermann G, Song H, Gage FH. Kempermann G, et al. Cold Spring Harb Perspect Biol. 2015 Sep 1;7(9):a018812. doi: 10.1101/cshperspect.a018812. Cold Spring Harb Perspect Biol. 2015. PMID: 26330519 Free PMC article. Review.
Cited by
- A Periodic Diet that Mimics Fasting Promotes Multi-System Regeneration, Enhanced Cognitive Performance, and Healthspan.
Brandhorst S, Choi IY, Wei M, Cheng CW, Sedrakyan S, Navarrete G, Dubeau L, Yap LP, Park R, Vinciguerra M, Di Biase S, Mirzaei H, Mirisola MG, Childress P, Ji L, Groshen S, Penna F, Odetti P, Perin L, Conti PS, Ikeno Y, Kennedy BK, Cohen P, Morgan TE, Dorff TB, Longo VD. Brandhorst S, et al. Cell Metab. 2015 Jul 7;22(1):86-99. doi: 10.1016/j.cmet.2015.05.012. Epub 2015 Jun 18. Cell Metab. 2015. PMID: 26094889 Free PMC article. Clinical Trial. - Human iPSC-Derived Hippocampal Spheroids: An Innovative Tool for Stratifying Alzheimer Disease Patient-Specific Cellular Phenotypes and Developing Therapies.
Pomeshchik Y, Klementieva O, Gil J, Martinsson I, Hansen MG, de Vries T, Sancho-Balsells A, Russ K, Savchenko E, Collin A, Vaz AR, Bagnoli S, Nacmias B, Rampon C, Sorbi S, Brites D, Marko-Varga G, Kokaia Z, Rezeli M, Gouras GK, Roybon L. Pomeshchik Y, et al. Stem Cell Reports. 2020 Jul 14;15(1):256-273. doi: 10.1016/j.stemcr.2020.06.001. Epub 2020 Jun 25. Stem Cell Reports. 2020. PMID: 32589876 Free PMC article. - Protective Effects of Early Caffeine Administration in Hyperoxia-Induced Neurotoxicity in the Juvenile Rat.
Heise J, Schmitz T, Bührer C, Endesfelder S. Heise J, et al. Antioxidants (Basel). 2023 Jan 28;12(2):295. doi: 10.3390/antiox12020295. Antioxidants (Basel). 2023. PMID: 36829854 Free PMC article. - Modifications of hippocampal circuits and early disruption of adult neurogenesis in the tg2576 mouse model of Alzheimer's disease.
Krezymon A, Richetin K, Halley H, Roybon L, Lassalle JM, Francès B, Verret L, Rampon C. Krezymon A, et al. PLoS One. 2013 Sep 27;8(9):e76497. doi: 10.1371/journal.pone.0076497. eCollection 2013. PLoS One. 2013. PMID: 24086745 Free PMC article. - Defects in the cerebella of conditional Neurod1 null mice correlate with effective Tg(Atoh1-cre) recombination and granule cell requirements for Neurod1 for differentiation.
Pan N, Jahan I, Lee JE, Fritzsch B. Pan N, et al. Cell Tissue Res. 2009 Sep;337(3):407-28. doi: 10.1007/s00441-009-0826-6. Epub 2009 Jul 17. Cell Tissue Res. 2009. PMID: 19609565 Free PMC article.
References
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. - PubMed
- Curtis MA, Kam M, Nannmark U, Anderson MF, Axell MZ, et al. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 2007;315:1243–1249. - PubMed
- Kempermann G, Jessberger S, Steiner B, Kronenberg G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004;27:447–452. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases