The emergence of lineage-specific chromosomal topologies from coordinate gene regulation - PubMed (original) (raw)
The emergence of lineage-specific chromosomal topologies from coordinate gene regulation
Indika Rajapakse et al. Proc Natl Acad Sci U S A. 2009.
Abstract
Although the importance of chromosome organization during mitosis is clear, it remains to be determined whether the nucleus assumes other functionally relevant chromosomal topologies. We have previously shown that homologous chromosomes have a tendency to associate during hematopoiesis according to their distribution of coregulated genes, suggesting cell-specific nuclear organization. Here, using the mathematical approaches of distance matrices and coupled oscillators, we model the dynamic relationship between gene expression and chromosomal associations during the differentiation of a multipotential hematopoietic progenitor. Our analysis reveals dramatic changes in total genomic order: Commitment of the progenitor results in an initial increase in entropy at both the level of gene coregulation and chromosomal organization, which we suggest represents a phase transition, followed by a progressive decline in entropy during differentiation. The stabilization of a highly ordered state in the differentiated cell types results in lineage-specific chromosomal topologies and is related to the emergence of coherence-or self-organization-between chromosomal associations and coordinate gene regulation. We discuss how these observations may be generally relevant to cell fate decisions encountered by progenitor/stem cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
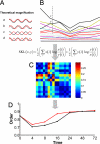
Fig. 1.
The order of coregulated gene expression networks increases during differentiation. (A) Potential differences between the expression patterns of 2 genes over time. (B) Pick any 2 genes (x and y) and measure the pairwise distance (relative entropy) during differentiation. (C) Example of a portion of the N × N distance matrix consisting of all pairwise comparisons at one time point. (D) Order is the fraction of genes at any given time point (0, 4, 8, 16, 24, 48, and 72 h) that are coregulated, thereby residing in a GC that reflects their hierarchical clustering. As genes entrain with or diverge from the expression pattern of the GC during the course of differentiation, the change in order as the system evolves can be determined. In the progenitor state (time 0), the GC corresponds to 84% of coregulated genes, is reduced to as low as 70% at 4 h, and reaches a maximum of 91% in the fully differentiated state. Red and black lines represent erythroid and neutrophil lineages, respectively.
Fig. 2.
Coregulated gene expression and chromosomal association networks are related. (A) Example of an erythroid rosette with each chromosome identified by using spectral karyotyping (SKY). (B) The chromosomal association network represented as a 19 × 19 frequency matrix counting all possible chromosome associations. (C) The larger coregulated gene expression network compressed to a 19 × 19 chromosomal association matrix, using the mean of coregulated genes found on each chromosome.
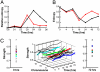
Fig. 3.
The organization of chromosomal associations increases during differentiation. (A) Graph of the relative entropy of chromosomal associations as computed from the SSDs for the progenitor against successive time points. (B) The general entropy (15) for each time point in the differentiation, normalized between 0 and 1. (C) Strength of chromosomal contribution to the chromosomal association network during differentiation. (Left) The front elevation at 0 h (the progenitor). (Right) The elevation from 72 h (erythroid). (Center) The total chromosomal contributions over time. Representative chromosomes 1, 6, 11, and 17 are indicated as green, blue, red, and dark blue, respectively.
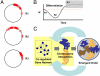
Fig. 4.
Chromosomes self-organize according to coordinate gene regulation. (A) Geometric interpretation of the chromosomal network, where the phases of θ_i_ are plotted on the unit circle. Three snapshots during differentiation are represented, where R1 is the distribution of the network representing the progenitor state, R2 the phase transition, and R3 the erythroid differentiated cell type. (B) Schematic illustration of the evolution of the order parameter (R) seen in numerical simulations of the chromosomal oscillator model for a specific coupling strength equation (19). (C) Schematic illustration for the mechanics of self-organization, with local interactions (gene coregulation) leading to chromosomal associations that emerge cooperatively in a cell-specific organization of the nucleus, which in turn feeds back to strengthen the local associations.
Comment in
- Self-organization in the genome.
Misteli T. Misteli T. Proc Natl Acad Sci U S A. 2009 Apr 28;106(17):6885-6. doi: 10.1073/pnas.0902010106. Epub 2009 Apr 22. Proc Natl Acad Sci U S A. 2009. PMID: 19416923 Free PMC article. No abstract available.
Similar articles
- Genomic Pangea: coordinate gene regulation and cell-specific chromosomal topologies.
Laster K, Kosak ST. Laster K, et al. Curr Opin Cell Biol. 2010 Jun;22(3):314-9. doi: 10.1016/j.ceb.2010.04.009. Epub 2010 May 22. Curr Opin Cell Biol. 2010. PMID: 20547047 - Coordinate gene regulation during hematopoiesis is related to genomic organization.
Kosak ST, Scalzo D, Alworth SV, Li F, Palmer S, Enver T, Lee JS, Groudine M. Kosak ST, et al. PLoS Biol. 2007 Nov;5(11):e309. doi: 10.1371/journal.pbio.0050309. PLoS Biol. 2007. PMID: 18031200 Free PMC article. - Linking Hematopoietic Differentiation to Co-Expressed Sets of Pluripotency-Associated and Imprinted Genes and to Regulatory microRNA-Transcription Factor Motifs.
Hamed M, Trumm J, Spaniol C, Sethi R, Irhimeh MR, Fuellen G, Paulsen M, Helms V. Hamed M, et al. PLoS One. 2017 Jan 4;12(1):e0166852. doi: 10.1371/journal.pone.0166852. eCollection 2017. PLoS One. 2017. PMID: 28052084 Free PMC article. - More to cohesin than meets the eye: complex diversity for fine-tuning of function.
Pezic D, Weeks SL, Hadjur S. Pezic D, et al. Curr Opin Genet Dev. 2017 Apr;43:93-100. doi: 10.1016/j.gde.2017.01.004. Epub 2017 Feb 9. Curr Opin Genet Dev. 2017. PMID: 28189962 Review. - Enhancers and their dynamics during hematopoietic differentiation and emerging strategies for therapeutic action.
Cico A, Andrieu-Soler C, Soler E. Cico A, et al. FEBS Lett. 2016 Nov;590(22):4084-4104. doi: 10.1002/1873-3468.12424. Epub 2016 Oct 6. FEBS Lett. 2016. PMID: 27645909 Review.
Cited by
- Reconstructing regulatory networks from the dynamic plasticity of gene expression by mutual information.
Wang J, Chen B, Wang Y, Wang N, Garbey M, Tran-Son-Tay R, Berceli SA, Wu R. Wang J, et al. Nucleic Acids Res. 2013 Apr;41(8):e97. doi: 10.1093/nar/gkt147. Epub 2013 Mar 6. Nucleic Acids Res. 2013. PMID: 23470995 Free PMC article. - Getting connected in the globin interactome.
Ragoczy T, Groudine M. Ragoczy T, et al. Nat Genet. 2010 Jan;42(1):16-7. doi: 10.1038/ng0110-16. Nat Genet. 2010. PMID: 20037614 - Distant cis-regulatory elements in human skeletal muscle differentiation.
McCord RP, Zhou VW, Yuh T, Bulyk ML. McCord RP, et al. Genomics. 2011 Dec;98(6):401-11. doi: 10.1016/j.ygeno.2011.08.003. Epub 2011 Aug 23. Genomics. 2011. PMID: 21907276 Free PMC article. - Finding Self-organization from the Dynamic Gene Expressions of Innate Immune Responses.
Selvarajoo K, Giuliani A. Selvarajoo K, et al. Front Physiol. 2012 Jun 11;3:192. doi: 10.3389/fphys.2012.00192. eCollection 2012. Front Physiol. 2012. PMID: 22701431 Free PMC article. No abstract available. - On emerging nuclear order.
Rajapakse I, Groudine M. Rajapakse I, et al. J Cell Biol. 2011 Mar 7;192(5):711-21. doi: 10.1083/jcb.201010129. J Cell Biol. 2011. PMID: 21383074 Free PMC article. Review.
References
- Kauffman SA. Emergent properties in random complex automata. Physica D. 1984;10(1–2):145–156.
- Langton CG. Computation at the edge of chaos: Phase transitions and emergent computation. Physica D. 1990;42(1–3):12–37.
- Newman MEJ, Barabasi AL, Watts DJ. The Structure and Dynamics of Networks. Princeton: Princeton Univ Press; 2006.
- Strogatz SH. Sync: The Emerging Science of Spontaneous Order. New York: Hyperion; 2003.
Publication types
MeSH terms
Grants and funding
- T32 CA80416/CA/NCI NIH HHS/United States
- R01 CA074841/CA/NCI NIH HHS/United States
- P01 CA53996/CA/NCI NIH HHS/United States
- P01 CA053996/CA/NCI NIH HHS/United States
- R01 HL65440/HL/NHLBI NIH HHS/United States
- R01 HL065440/HL/NHLBI NIH HHS/United States
- R01 CA 074841/CA/NCI NIH HHS/United States
- R37 DK044746/DK/NIDDK NIH HHS/United States
- T32 CA080416/CA/NCI NIH HHS/United States
- R37 DK44746/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources