TGF-beta1 dampens the susceptibility of dendritic cells to environmental stimulation, leading to the requirement for danger signals for activation - PubMed (original) (raw)
TGF-beta1 dampens the susceptibility of dendritic cells to environmental stimulation, leading to the requirement for danger signals for activation
Tomoyuki Ohtani et al. Immunology. 2009 Apr.
Abstract
In contrast to its favourable effects on Langerhans cell (LC) differentiation, transforming growth factor (TGF)-beta1 has been reported to prevent dendritic cells from maturing in response to tumour necrosis factor (TNF)-alpha, interleukin (IL)-1beta, or lipopolysaccharide (LPS). We first characterized the effects of TGF-beta1 on dendritic cell function by testing the response of TGF-beta1-treated monocyte-derived dendritic cells (MoDCs) to maturation stimuli that LCs receive in the epidermis, namely, haptens, ATP and ultraviolet (UV). TGF-beta1 treatment, which augmented E-cadherin and down-regulated dendritic cell-specific ICAM3-grabbing non-integrin on MoDCs, significantly suppressed their CD86 expression and hapten-induced expression of IL-1beta and TNF-alpha mRNA and protein. As TGF-beta1-treated MoDCs lacked Langerin expression, we demonstrated the suppressive effects of TGF-beta1 on haematopoietic progenitor cell-derived dendritic cells expressing both CD1a and Langerin. These suppressive effects of TGF-beta1 increased with the duration of treatment. Furthermore, TGF-beta1-treated MoDCs became resistant to apoptosis/necrosis induced by high hapten, ATP or UV doses. This was mainly attributable to dampened activation of p38 mitogen-activated protein kinase (MAPK) in TGF-beta1-treated MoDCs. Notably, although ATP or hapten alone could only induce CD86 expression weakly and could not augment the allogeneic T-cell stimulatory function of TGF-beta1-treated MoDCs, ATP and hapten synergized to stimulate these phenotypic and functional changes. Similarly, 2,4-dinitro, 1-chlorobenzene (DNCB) augmented the maturation of TGF-beta1-treated MoDCs upon co-culture with a keratinocyte cell line, in which ATP released by the hapten-stimulated keratinocytes synergized with the hapten to induce their maturation. These data may suggest that TGF-beta1 protects LCs from being overactivated by harmless environmental stimulation, while maintaining their ability to become activated in response to danger signals released by keratinocytes.
Figures
Figure 1
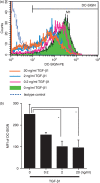
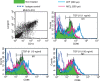
Dendritic cell-specific ICAM3-grabbing non-integrin (DC-SIGN) expression by monocyte-derived dendritic cells (MoDCs) is suppressed by transforming growth factor (TGF)-β1 treatment. CD14+ monocytes were cultured in complete medium containing 100 ng/ml each of granulocyte–macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-4 and different concentrations of TGF-β1. After 6 days of culture, DC-SIGN expression was analysed by flow cytometry. (a) Representative data from three independent experiments; (b) the summarized data. The mean fluorescence intensity (MFI) ± standard error of the mean from three independent experiments is shown. *Means P < 0·05 by paired _t-_test. PE, phycoerythrin.
Figure 2
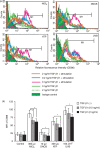
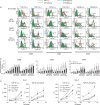
Stimulation of monocyte-derived dendritic cells (MoDCs) with haptens, ATP or ultraviolet B (UVB) does not augment CD86 expression when transforming growth factor (TGF)-β1 is present. CD14+ monocytes were cultured in complete medium containing 100 ng/ml each of granulocyte–macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-4 and different concentrations of TGF-β1. After 6 days of culture, they were stimulated for 48 hr with the indicated concentrations of NiCl2, 2,4-dinitro, 1-chlorobenzene (DNCB) and ATP and the optimal dose of UVB and then analysed for their expression of CD86 by flow cytometry. Representative data from six independent experiments are shown in (a) and the data are summarized in (b). Mean fluorescence intensity (MFI) ± standard error of the mean from six independent experiments is shown. *Means P < 0·05 by paired _t-_test.
Figure 3
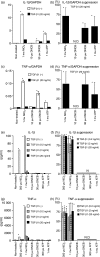
Stimulation of monocyte-derived dendritic cells (MoDCs) with haptens, ATP or ultraviolet B (UVB) does not augment cytokine mRNA and protein production when transforming growth factor (TGF)-β1 is present. CD14+ monocytes were cultured in complete medium containing 100 ng/ml each of granulocyte–macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-4 and different concentrations of TGF-β1. After 6 days of culture, they were stimulated with the indicated concentrations of NiCl2, 2,4-dinitro, 1-chlorobenzene (DNCB) and ATP. After 6 hr of culture, the IL-1β GAPDH and tumour necrosis factor (TNF)-α/GAPDH mRNA ratios were determined by real-time polymerase chain reaction (PCR). Representative data from three independent experiments are shown in (a) and (c). Results are presented as the mean ± standard deviation obtained from triplicate cultures. *Means P < 0·05 by unpaired Student’s _t-_test. The data are summarized in (b) and (d). The mean per cent suppression ± standard error of the mean (SEM) for three independent experiments is shown. *P<0·05 by paired _t-_test. After 48 hr of culture, the concentrations of IL-1β and TNF-α in the culture supernatants were examined by enzyme-linked immunosorbent assay (ELISA). Representative data from three independent experiments are shown in (e) and (g). Results are presented as the mean ± standard deviation obtained from triplicate cultures. *Means P < 0·05 by unpaired Student’s _t-_test. The data from these experiments are summarized in (f) and (h). The mean per cent suppression ± SEM for three independent experiments is shown. *P<0·05 by paired _t-_test. ND, not detected.
Figure 4
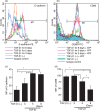
The ability of ATP to stimulate monocyte-derived dendritic cell (MoDC) CD86 expression decreases the longer the cells have been co-cultured with transforming growth factor (TGF)-β1. Monocytes were cultured with granulocyte–macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-4 and 20 ng/ml TGF-β1 was added at different time intervals after the initiation of the culture. After 6 days of culture, the E-cadherin expression of some of the cells was examined by flow cytometry (a). The remaining cells were stimulated with 300 μ
m
ATP for 2 days and their CD86 expression was determined by flow cytometry (b). Representative data from three different experiments are shown. Summarized data from three different experiments for the change in relative fluorescence intensity are shown in (c) and (d). The mean fluorescence intensity ± standard error of the mean from three independent experiments is shown. *Means P<0·05 by paired _t-_test. FITC, fluorescein isothiocyanate; PE, phycoerythrin.
Figure 5
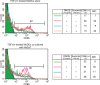
Transforming growth factor (TGF)-β1 suppresses CD86 expression by Langerin+ haematopoietic progenitor cell (HPC)-derived dendritic cells (DCs) stimulated with ATP or NiCl2. CD34+ HPCs were cultured in X-VIVO 15 medium in 24-well tissue culture plates supplemented with 100 ng/ml macrophage colony-stimulating factor (GM-CSF), in conjunction with a combination of stem cell factor (SCF), Flt3 ligand (Flt3L), tumour necrosis factor (TNF)-α, and various concentrations of TGF-β1 for 10–14 days. Cell surface and intracytoplasmic Langerin expression by CD34+ HPC-derived DCs cultured in the presence of 1 ng/ml of TGF-β1 was examined by flow cytometry (a). The CD86 expression of HPC-derived DCs cultured in the presence of 1 ng/ml (b), 10 ng/ml (c), and 100 ng/ml (d) of TGF-β1 was examined 48 hr after stimulation with 300 μ
m
ATP or 300 μ
m
NiCl2. Representative data from three different experiments are shown.
Figure 6
Transforming growth factor (TGF)-β1-treated monocyte-derived dendritic cells (MoDCs) are resistant to the apoptosis or necrosis that is induced by high concentrations of haptens or ATP. CD14+ monocytes were cultured with granulocyte–macrophage colony-stimulating factor (GM-CSF), interleukin (IL)-4 and different concentrations of TGF-β1 for 6 days and then stimulated with 1 or 3 m
m
NiCl2, 30 μ
m
2,4-dinitro, 1-chlorobenzene (DNCB), 1 or 3 m
m
ATP, or 200 J/m2 of UVB. After 12 hr of culture, the apoptotic cells (Annexin V-positive and PI-negative cells) and necrotic cells (PI-positive cells) were then quantified by flow cytometry by double staining with fluorescein isothiocyanate (FITC)-Annexin V and propidium iodide. The data represent three different experiments.
Figure 7
Transforming growth factor (TGF)-β1 treatment of monocyte-derived dendritic cells (MoDCs) suppresses the phosphorylation of p38 mitogen-activated protein kinases (MAPK) induced by haptens, ATP or ultraviolet B (UVB). CD14+ monocytes were cultured with granulocyte–macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-4 with or without 20 ng/ml TGF-β1 for 6 days and then stimulated with the indicated NiCl2, 2,4-dinitro, 1-chlorobenzene (DNCB) and ATP concentrations (a and b) or the indicated UVB dose (c and d). After 30 min, the levels of phosphorylated and unphosphorylated p42/44 extracellular signal-regulated kinases (ERKs), stress-activated protein kinases/ c-JUN N-terminal kinase (SAPK/JNK) p38 MAPK and protein kinase B (Akt) were examined using immunoblotting kits (a and c). These data were also analysed by densitometry (b and d). Representative data from three different experiments are shown.
Figure 8
ATP and haptens synergistically augment the CD86 expression of monocyte-derived dendritic cells (MoDCs) and their antigen-presenting function only when the MoDCs have been treated with transforming growth factor (TGF)-β1. CD14+ monocytes were cultured with granulocyte–macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-4 with or without 20 ng/ml TGF-β1 for 6 days and then stimulated for 48 hr with graded concentrations of ATP along with the indicated concentrations of NiCl2, 2,4-dinitro, 1-chlorobenzene (DNCB) or tumour necrosis factor (TNF)-α. The CD86, CD80 and CD83 expression of the cells was examined by flow cytometry. Representative data from four independent experiments are shown in (a). The data are summarized in (b). The mean fluorescence intensity ± standard error of the mean (SEM) from three independent experiments is shown. In the mixed lymphocyte reaction (MLR), graded numbers of MoDCs or TGF-β1-treated MoDCs stimulated with NiCl2 (300 μ
m
) or ATP (300 μ
m
) or both NiCl2 and ATP together were cultured with 105 allogeneic T cells in 96-well flat-bottom culture plates. After 5 days in the MLR assay, the cells were pulse-labelled for the last 16 hr with [3H]thymidine. Results are presented as the mean ± standard deviation obtained from triplicate cultures in (c). *Means P < 0·05 by unpaired Student’s _t-_test. c.p.m., counts per minute; FITC, fluorescein isothiocyanate; PE, phycoerythrin.
Figure 9
Co-culture with keratinocytes markedly augments the 2,4-dinitro, 1-chlorobenzene (DNCB)-induced CD86 expression of transforming growth factor (TGF)-β1-treated monocyte-derived dendritic cells (MoDCs). TGF-β1-treated MoDCs were co-cultured with the keratinocyte cell line HaCaT for 12 hr and then treated or not treated with the ATP antagonist suramin for 1 hr before culture with DNCB for 48 hr. The CD86 expression of the TGF-β1-treated MoDCs was then examined by flow cytometry. The data represent two independent experiments. MFI, mean fluorescence intensity.
Similar articles
- Dendritic cells differently respond to haptens and irritants by their production of cytokines and expression of co-stimulatory molecules.
Aiba S, Terunuma A, Manome H, Tagami H. Aiba S, et al. Eur J Immunol. 1997 Nov;27(11):3031-8. doi: 10.1002/eji.1830271141. Eur J Immunol. 1997. PMID: 9394834 - Functional Langerinhigh-Expressing Langerhans-like Cells Can Arise from CD14highCD16- Human Blood Monocytes in Serum-Free Condition.
Picarda G, Chéneau C, Humbert JM, Bériou G, Pilet P, Martin J, Duteille F, Perrot P, Bellier-Waast F, Heslan M, Haspot F, Guillon F, Josien R, Halary FA. Picarda G, et al. J Immunol. 2016 May 1;196(9):3716-28. doi: 10.4049/jimmunol.1501304. Epub 2016 Mar 25. J Immunol. 2016. PMID: 27016604 - The mucosal factors retinoic acid and TGF-β1 induce phenotypically and functionally distinct dendritic cell types.
den Hartog G, van Altena C, Savelkoul HF, van Neerven RJ. den Hartog G, et al. Int Arch Allergy Immunol. 2013;162(3):225-36. doi: 10.1159/000353243. Epub 2013 Sep 6. Int Arch Allergy Immunol. 2013. PMID: 24022014 - Notch-Mediated Generation of Monocyte-Derived Langerhans Cells: Phenotype and Function.
Bellmann L, Zelle-Rieser C, Milne P, Resteu A, Tripp CH, Hermann-Kleiter N, Zaderer V, Wilflingseder D, Hörtnagl P, Theochari M, Schulze J, Rentzsch M, Del Frari B, Collin M, Rademacher C, Romani N, Stoitzner P. Bellmann L, et al. J Invest Dermatol. 2021 Jan;141(1):84-94.e6. doi: 10.1016/j.jid.2020.05.098. Epub 2020 Jun 6. J Invest Dermatol. 2021. PMID: 32522485 Free PMC article. Review.
Cited by
- Severe dermatitis with loss of epidermal Langerhans cells in human and mouse zinc deficiency.
Kawamura T, Ogawa Y, Nakamura Y, Nakamizo S, Ohta Y, Nakano H, Kabashima K, Katayama I, Koizumi S, Kodama T, Nakao A, Shimada S. Kawamura T, et al. J Clin Invest. 2012 Feb;122(2):722-32. doi: 10.1172/JCI58618. Epub 2012 Jan 3. J Clin Invest. 2012. PMID: 22214844 Free PMC article. - Tumor-promoting role of TGFβ1 signaling in ultraviolet B-induced skin carcinogenesis is associated with cutaneous inflammation and lymph node migration of dermal dendritic cells.
Ravindran A, Mohammed J, Gunderson AJ, Cui X, Glick AB. Ravindran A, et al. Carcinogenesis. 2014 Apr;35(4):959-66. doi: 10.1093/carcin/bgt486. Epub 2013 Dec 20. Carcinogenesis. 2014. PMID: 24363069 Free PMC article. - Therapeutic targeting of TGF-β in cancer: hacking a master switch of immune suppression.
van den Bulk J, de Miranda NFCC, Ten Dijke P. van den Bulk J, et al. Clin Sci (Lond). 2021 Jan 15;135(1):35-52. doi: 10.1042/CS20201236. Clin Sci (Lond). 2021. PMID: 33399850 Free PMC article. Review. - Blockade of TGF-β signaling reactivates HIV-1/SIV reservoirs and immune responses in vivo.
Samer S, Thomas Y, Araínga M, Carter C, Shirreff LM, Arif MS, Avita JM, Frank I, McRaven MD, Thuruthiyil CT, Heybeli VB, Anderson MR, Owen B, Gaisin A, Bose D, Simons LM, Hultquist JF, Arthos J, Cicala C, Sereti I, Santangelo PJ, Lorenzo-Redondo R, Hope TJ, Villinger FJ, Martinelli E. Samer S, et al. JCI Insight. 2022 Nov 8;7(21):e162290. doi: 10.1172/jci.insight.162290. JCI Insight. 2022. PMID: 36125890 Free PMC article. - Secondary lymphoid organ homing phenotype of human myeloid dendritic cells disrupted by an intracellular oral pathogen.
Miles B, Zakhary I, El-Awady A, Scisci E, Carrion J, O'Neill JC, Rawlings A, Stern JK, Susin C, Cutler CW. Miles B, et al. Infect Immun. 2014 Jan;82(1):101-11. doi: 10.1128/IAI.01157-13. Epub 2013 Oct 14. Infect Immun. 2014. PMID: 24126519 Free PMC article.
References
- Strobl H, Riedl E, Scheinecker C, Bello-Fernandez C, Pickl WF, Rappersberger K, Majdic O, Knapp W. TGF-beta 1 promotes in vitro development of dendritic cells from CD34+ hematopoietic progenitors. J Immunol. 1996;157:1499–507. - PubMed
- Riedl E, Strobl H, Majdic O, Knapp W. TGF-beta 1 promotes in vitro generation of dendritic cells by protecting progenitor cells from apoptosis. J Immunol. 1997;158:1591–7. - PubMed
- Strobl H, Bello-Fernandez C, Riedl E, Pickl WF, Majdic O, Lyman SD, Knapp W. Flt3 ligand in cooperation with transforming growth factor-beta1 potentiates in vitro development of langerhans-type dendritic cells and allows single-cell dendritic cell cluster formation under serum-free conditions. Blood. 1997;90:1425–34. - PubMed
- Caux C, Massacrier C, Dubois B, Valladeau J, Dezutter-Dambuyant C, Durand I, Schmitt D, Saeland S. Respective involvement of TGF-beta and IL-4 in the development of Langerhans cells and non-Langerhans dendritic cells from CD34+ progenitors. J Leukocyte Biol. 1999;66:781–91. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials