Arachidonic acid, ARC channels, and Orai proteins - PubMed (original) (raw)
Review
Arachidonic acid, ARC channels, and Orai proteins
Trevor J Shuttleworth. Cell Calcium. 2009 Jun.
Abstract
A critical role for arachidonic acid in the regulation of calcium entry during agonist activation of calcium signals has become increasingly apparent in numerous studies over the past 10 years or so. In particular, low concentrations of this fatty acid, generated as a result of physiologically relevant activation of appropriate receptors, induces the activation of a unique, highly calcium-selective conductance now known as the ARC channel. Activation of this channel is specifically dependent on arachidonic acid acting at the intracellular surface of the membrane, and is entirely independent of any depletion of internal calcium stores. Importantly, a specific role of this channel in modulating the frequency of oscillatory calcium signals in various cell types has been described. Recent studies, subsequent to the discovery of STIM1 and the Orai proteins and their role in the store-operated CRAC channels, have revealed that these same proteins are also integral components of the ARC channels and their activation. However, unlike the CRAC channels, activation of the ARC channels depends on the pool of STIM1 that is constitutively resident in the plasma membrane, and the pore of these channels is comprised of both Orai1 and Orai3 subunits. The clear implication is that CRAC channels and ARC channels are closely related, but have evolved to play unique roles in the modulation of calcium signals-largely as a result of their entirely distinct modes of activation. Given this, although the precise details of how arachidonic acid acts to activate the channels remain unclear, it seems likely that the specific molecular features of these channels that distinguish them from the CRAC channels--namely Orai3 and/or plasma membrane STIM1--will be involved.
Figures
Figure 1
Biophysical and pharmacological characteristics of the ARC channels. (A) Activation of ARC channel currents in a HEK293 cell by exogenous addition of arachidonic acid. Inward currents were measured at −80mV. Arachidonic acid (8 µM) was added to the external medium where indicated (arrowhead). (B) Current-voltage relationship of the arachidonic acid-induced currents in HEK293 cells. (C) Effect of gadolinium on ARC channel currents. Mean (± SE) ARC channel current-voltage relationships are shown for control cells (black symbols), and in cells exposed to Gd3+ (5 µM) (grey symbols). (D) Absence of any fast-inactivation in ARC channel currents. Shown is a representative recording of ARC channel current during a brief (250 ms) pulse to −80 mV. (E) Lack of any significant effect of 2-APB on ARC channel currents. Mean (± SE) ARC channel current-voltage relationships are shown for control cells (black symbols), and in cells exposed to 2-APB (100 µM) (grey symbols). Data in (C) and (E) redrawn from [14].
Figure 2
The involvement of plasma membrane STIM1 and Orai proteins in ARC channel function. (A) Western blot showing STIM1 protein in control cells (lane 1), in cells transfected with the STIM1 siRNA (lane 2), and in siRNA-transfected cells expressing the siRNA-resistant _N_-glycosylation mutant STIM1 (lane 3). In the latter, the residual endogenous STIM1 after siRNA treatment can be seen as a faint band running at the same point as in lanes 1 and 2, whilst the expressed siRNA-resistant glycosylation mutant runs at a lightly lower molecular weight. (B) Expression of the _N_-glycosylation mutant STIM1 specifically inhibits ARC channel currents. Values represent mean (± SE) inward CRAC channel and ARC channel current densities measured at −80 mV in control cells (white bars), and in siRNA-treated cells expressing the _N_-glycosylation mutant STIM1 (black bars), n = 6–11. (C) Expression of the dominant-negative Orai1 inhibits both CRAC and ARC channel currents, whilst expression of the dominant-negative Orai3 inhibits only the ARC channel currents. Values represent mean (± SE) inward current densities measured at −80 mV in cells stably expressing STIM1 (white bars), and in the same cells transfected with the E106Q mutant Orai1 (grey bars), or the E81Q mutant Orai3 (black bars), n = 4–6. Data redrawn from [24] and [25].
Figure 3
(A) Mean (±SE) inward current densities measured at −80 mV induced by exogenous application of the diacylglycerol OAG (100 µM, grey bar), and various fatty acids (black bars, all at 8 µM), n = 4–11. (B) ARC channel current magnitudes induced by different concentrations of exogenous arachidonic acid. Values are mean (± SE) inward current density at −80 mV (n = 4–6). (C) Comparison of internally and externally applied arachidonyl coenzyme A on currents through ARC channels. Shown are the mean (± SE) current-voltage relationships of the currents activated by application of arachidonyl coenzyme A (8 µM) added in the pipette solution (black symbols, n = 6), or in the external bath (grey symbols, n = 5). Data in (B) and (C) redrawn from [14].
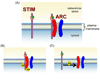
Figure 4
Potential sites of arachidonic acid action in the activation of ARC channels. (A) The unique features of ARC channel activity include the presence of Orai3 (red), along with Orai1 (blue), as the pore forming components of the channel itself, and the specific dependence on the pool of STIM1 that is resident in the plasma membrane. Significantly, the extracellular location of the N-terminal EF hand of this plasma membrane STIM1 means that it would, under normal circumstances, always have calcium (green) bound to it. (B) Based on these unique components, activation of the channels is likely to involve arachidonic acid (aa) acting on the plasma membrane STIM1, and/or on the Orai3 protein of the channel. (C) Alternatively, arachidonic acid may act to induce an interaction between these proteins.
Similar articles
- Store-Independent Orai Channels Regulated by STIM.
Zhang X, Gueguinou M, Trebak M. Zhang X, et al. In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 11. In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 11. PMID: 30299650 Free Books & Documents. Review. - The ARC channel--an endogenous store-independent Orai channel.
Thompson JL, Mignen O, Shuttleworth TJ. Thompson JL, et al. Curr Top Membr. 2013;71:125-48. doi: 10.1016/B978-0-12-407870-3.00006-8. Curr Top Membr. 2013. PMID: 23890114 - Both Orai1 and Orai3 are essential components of the arachidonate-regulated Ca2+-selective (ARC) channels.
Mignen O, Thompson JL, Shuttleworth TJ. Mignen O, et al. J Physiol. 2008 Jan 1;586(1):185-95. doi: 10.1113/jphysiol.2007.146258. Epub 2007 Nov 8. J Physiol. 2008. PMID: 17991693 Free PMC article. - The molecular architecture of the arachidonate-regulated Ca2+-selective ARC channel is a pentameric assembly of Orai1 and Orai3 subunits.
Mignen O, Thompson JL, Shuttleworth TJ. Mignen O, et al. J Physiol. 2009 Sep 1;587(Pt 17):4181-97. doi: 10.1113/jphysiol.2009.174193. Epub 2009 Jul 21. J Physiol. 2009. PMID: 19622606 Free PMC article. - STIM and Orai proteins and the non-capacitative ARC channels.
Shuttleworth TJ. Shuttleworth TJ. Front Biosci (Landmark Ed). 2012 Jan 1;17(3):847-60. doi: 10.2741/3960. Front Biosci (Landmark Ed). 2012. PMID: 22201777 Free PMC article. Review.
Cited by
- Arachidonate-regulated Ca(2+) influx in human airway smooth muscle.
Thompson MA, Prakash YS, Pabelick CM. Thompson MA, et al. Am J Respir Cell Mol Biol. 2014 Jul;51(1):68-76. doi: 10.1165/rcmb.2013-0144OC. Am J Respir Cell Mol Biol. 2014. PMID: 24471656 Free PMC article. - The role of Orai-STIM calcium channels in melanocytes and melanoma.
Stanisz H, Vultur A, Herlyn M, Roesch A, Bogeski I. Stanisz H, et al. J Physiol. 2016 Jun 1;594(11):2825-35. doi: 10.1113/JP271141. Epub 2016 Apr 6. J Physiol. 2016. PMID: 26864956 Free PMC article. Review. - Calcium in tumour metastasis: new roles for known actors.
Prevarskaya N, Skryma R, Shuba Y. Prevarskaya N, et al. Nat Rev Cancer. 2011 Jul 22;11(8):609-18. doi: 10.1038/nrc3105. Nat Rev Cancer. 2011. PMID: 21779011 Review. - Orai3 is an estrogen receptor α-regulated Ca²⁺ channel that promotes tumorigenesis.
Motiani RK, Zhang X, Harmon KE, Keller RS, Matrougui K, Bennett JA, Trebak M. Motiani RK, et al. FASEB J. 2013 Jan;27(1):63-75. doi: 10.1096/fj.12-213801. Epub 2012 Sep 19. FASEB J. 2013. PMID: 22993197 Free PMC article. - Alternative translation initiation gives rise to two isoforms of Orai1 with distinct plasma membrane mobilities.
Fukushima M, Tomita T, Janoshazi A, Putney JW. Fukushima M, et al. J Cell Sci. 2012 Sep 15;125(Pt 18):4354-61. doi: 10.1242/jcs.104919. Epub 2012 May 28. J Cell Sci. 2012. PMID: 22641696 Free PMC article.
References
- Putney JW., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. - PubMed
- Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353–356. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DE016999-05/DE/NIDCR NIH HHS/United States
- R01 GM040457-19/GM/NIGMS NIH HHS/United States
- R01 DE016999-04/DE/NIDCR NIH HHS/United States
- R01 GM040457-18/GM/NIGMS NIH HHS/United States
- DE 016999/DE/NIDCR NIH HHS/United States
- R01 GM040457/GM/NIGMS NIH HHS/United States
- R01 DE016999/DE/NIDCR NIH HHS/United States
- GM 040457/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous