Safety and efficacy of quinacrine in human prion disease (PRION-1 study): a patient-preference trial - PubMed (original) (raw)
Clinical Trial
doi: 10.1016/S1474-4422(09)70049-3. Epub 2009 Mar 9.
Michele Gorham, Fleur Hudson, Angus Kennedy, Geraldine Keogh, Suvankar Pal, Martin Rossor, Peter Rudge, Durre Siddique, Moira Spyer, Dafydd Thomas, Sarah Walker, Tom Webb, Steve Wroe, Janet Darbyshire
Affiliations
- PMID: 19278902
- PMCID: PMC2660392
- DOI: 10.1016/S1474-4422(09)70049-3
Clinical Trial
Safety and efficacy of quinacrine in human prion disease (PRION-1 study): a patient-preference trial
John Collinge et al. Lancet Neurol. 2009 Apr.
Abstract
Background: The propagation of prions, the causative agents of Creutzfeldt-Jakob disease and other human prion diseases, requires post-translational conversion of normal cellular prion protein to disease-associated forms. The antimalarial drug quinacrine (mepacrine) prevents this conversion in vitro, and was given to patients with various prion diseases to assess its safety and efficacy in changing the course of these invariably fatal and untreatable diseases.
Methods: Patients with prion disease were recruited via the UK national referral system and were offered a choice between quinacrine (300 mg daily), no quinacrine, or randomisation to immediate quinacrine or deferred quinacrine in an open-label, patient-preference trial. The primary endpoints were death and serious adverse events possibly or probably related to the study drug. This study is registered, ISRCTN 06722585.
Findings: 107 patients with prion disease (45 sporadic, two iatrogenic, 18 variant, and 42 inherited) were enrolled, 23 in a pilot study and 84 in the main study. Only two patients chose randomisation; 40 took quinacrine during follow-up (37 who chose it at enrollment). Choice of treatment was associated with disease severity, with those least and most severely affected more likely to choose not to receive quinacrine. 78 (73%) patients died: one randomly assigned to deferred treatment, 26 of 38 who chose immediate quinacrine, and 51 of 68 who chose no quinacrine. Although adjusted mortality was lower in those who chose to take quinacrine than in those who did not, this was due to confounding with disease severity, and there was no difference in mortality between groups after adjustment. Four of 40 patients who took quinacrine had a transient response on neurological rating scales. Only two of 14 reported serious adverse events were judged quinacrine-related.
Interpretation: Quinacrine at a dose of 300 mg per day was reasonably tolerated but did not significantly affect the clinical course of prion diseases in this observational study.
Figures
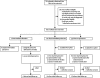
Figure 1
Trial profile *Relative with symptomatic inherited prion disease or recipient of blood transfusion. †From September, 2001–June, 2002, six patients received open-label quinacrine in an initial pilot study, and from August, 2002, to March, 2004, 17 more were offered quinacrine or no quinacrine in an extended pilot study (total 23 patients). ‡One originally chose not to take quinacrine but later agreed to randomisation 9 weeks after enrolment and was allocated immediate quinacrine.
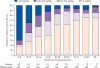
Figure 2
Quinacrine use after initiation
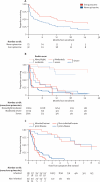
Figure 3
Survival Unadjusted survival from enrolment (A). Survival from enrolment by baseline Rankin score (B). Survival from first symptoms by type of human prion disease (C).
Comment in
- Clinical trials for prion disease: difficult challenges, but hope for the future.
Geschwind MD. Geschwind MD. Lancet Neurol. 2009 Apr;8(4):304-6. doi: 10.1016/S1474-4422(09)70050-X. Epub 2009 Mar 9. Lancet Neurol. 2009. PMID: 19278901 Free PMC article. No abstract available. - Clinical trials and methodological problems in prion diseases.
Puopolo M, Pocchiari M, Petrini C. Puopolo M, et al. Lancet Neurol. 2009 Sep;8(9):782; author reply 782-3. doi: 10.1016/S1474-4422(09)70214-5. Lancet Neurol. 2009. PMID: 19679270 No abstract available.
Similar articles
- PRION-1 scales analysis supports use of functional outcome measures in prion disease.
Mead S, Ranopa M, Gopalakrishnan GS, Thompson AG, Rudge P, Wroe S, Kennedy A, Hudson F, MacKay A, Darbyshire JH, Collinge J, Walker AS. Mead S, et al. Neurology. 2011 Nov 1;77(18):1674-83. doi: 10.1212/WNL.0b013e3182364890. Epub 2011 Oct 19. Neurology. 2011. PMID: 22013183 Free PMC article. - Quinacrine treatment trial for sporadic Creutzfeldt-Jakob disease.
Geschwind MD, Kuo AL, Wong KS, Haman A, Devereux G, Raudabaugh BJ, Johnson DY, Torres-Chae CC, Finley R, Garcia P, Thai JN, Cheng HQ, Neuhaus JM, Forner SA, Duncan JL, Possin KL, Dearmond SJ, Prusiner SB, Miller BL. Geschwind MD, et al. Neurology. 2013 Dec 3;81(23):2015-23. doi: 10.1212/WNL.0b013e3182a9f3b4. Epub 2013 Oct 11. Neurology. 2013. PMID: 24122181 Free PMC article. Clinical Trial. - Continuous quinacrine treatment results in the formation of drug-resistant prions.
Ghaemmaghami S, Ahn M, Lessard P, Giles K, Legname G, DeArmond SJ, Prusiner SB. Ghaemmaghami S, et al. PLoS Pathog. 2009 Nov;5(11):e1000673. doi: 10.1371/journal.ppat.1000673. Epub 2009 Nov 26. PLoS Pathog. 2009. PMID: 19956709 Free PMC article. - [Prospects of the therapeutic approaches to Creutzfeldt-Jakob disease: a clinical trial of antimalarial, quinacrine].
Furukawa H, Takahashi M, Nakajima M, Yamada T. Furukawa H, et al. Nihon Rinsho. 2002 Aug;60(8):1649-57. Nihon Rinsho. 2002. PMID: 12187766 Review. Japanese. - [Therapeutics for prion diseases].
Doh-ura K. Doh-ura K. Rinsho Shinkeigaku. 2003 Nov;43(11):820-2. Rinsho Shinkeigaku. 2003. PMID: 15152474 Review. Japanese.
Cited by
- Quinacrine induced mood disturbance--the unmasking of bipolar affective disorder.
Lally J, Byrne F, Walsh E. Lally J, et al. BMJ Case Rep. 2012 Jun 21;2012:bcr0220125842. doi: 10.1136/bcr.02.2012.5842. BMJ Case Rep. 2012. PMID: 22729334 Free PMC article. - Screening of Anti-Prion Compounds Using the Protein Misfolding Cyclic Amplification Technology.
Pritzkow S, Schauer I, Tupaki-Sreepurna A, Morales R, Soto C. Pritzkow S, et al. Biomolecules. 2024 Sep 4;14(9):1113. doi: 10.3390/biom14091113. Biomolecules. 2024. PMID: 39334879 Free PMC article. - PrPC knockdown by liposome-siRNA-peptide complexes (LSPCs) prolongs survival and normal behavior of prion-infected mice immunotolerant to treatment.
Bender H, Noyes N, Annis JL, Hitpas A, Mollnow L, Croak K, Kane S, Wagner K, Dow S, Zabel M. Bender H, et al. PLoS One. 2019 Jul 22;14(7):e0219995. doi: 10.1371/journal.pone.0219995. eCollection 2019. PLoS One. 2019. PMID: 31329627 Free PMC article. - Development of prognostic models for survival and care status in sporadic Creutzfeldt-Jakob disease.
Nihat A, Ranson JM, Harris D, McNiven K, Mok T, Rudge P, Collinge J, Llewellyn DJ, Mead S. Nihat A, et al. Brain Commun. 2022 Aug 2;4(4):fcac201. doi: 10.1093/braincomms/fcac201. eCollection 2022. Brain Commun. 2022. PMID: 35974795 Free PMC article. - A Single Subcutaneous Injection of Cellulose Ethers Administered Long before Infection Confers Sustained Protection against Prion Diseases in Rodents.
Teruya K, Oguma A, Nishizawa K, Kawata M, Sakasegawa Y, Kamitakahara H, Doh-Ura K. Teruya K, et al. PLoS Pathog. 2016 Dec 14;12(12):e1006045. doi: 10.1371/journal.ppat.1006045. eCollection 2016 Dec. PLoS Pathog. 2016. PMID: 27973536 Free PMC article.
References
- Collinge J. Prion diseases of humans and animals: their causes and molecular basis. Annu Rev Neurosci. 2001;24:519–550. - PubMed
- Wroe SJ, Pal S, Siddique D. Clinical presentation and pre-mortem diagnosis of variant Creutzfeldt-Jakob disease associated with blood transfusion: a case report. Lancet. 2006;368:2061–2067. - PubMed
- Mallucci G, Collinge J. Rational targeting for prion therapeutics. Nat Rev Neurosci. 2005;6:23–34. - PubMed
- Trevitt C, Collinge J. A systematic review of prion therapeutics in experimental models. Brain. 2006;129:2241–2265. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources