When the endogenous hallucinogenic trace amine N,N-dimethyltryptamine meets the sigma-1 receptor - PubMed (original) (raw)
Review
When the endogenous hallucinogenic trace amine N,N-dimethyltryptamine meets the sigma-1 receptor
Tsung-Ping Su et al. Sci Signal. 2009.
Abstract
N,N-dimethyltryptamine (DMT) is a hallucinogen found endogenously in human brain that is commonly recognized to target the 5-hydroxytryptamine 2A receptor or the trace amine-associated receptor to exert its psychedelic effect. DMT has been recently shown to bind sigma-1 receptors, which are ligand-regulated molecular chaperones whose function includes inhibiting various voltage-sensitive ion channels. Thus, it is possible that the psychedelic action of DMT might be mediated in part through sigma-1 receptors. Here, we present a hypothetical signaling scheme that might be triggered by the binding of DMT to sigma-1 receptors.
Figures
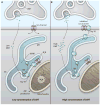
Fig. 1
Hypothetical scheme illustrating the signaling of N,_N_-dimethyltryptamine through sigma-1 receptors. (A) Sigma-1 receptors (Sig-1Rs) at the mitochondrion-associated endoplasmic reticulum (ER) membrane (MAM) function as ligand-activated molecular chaperones, particularly when ligands are present at concentrations close to their affinities (34). Sig-1R ligands, including DMT, at concentrations close to their Ki values, cause the dissociation of Sig-1Rs from another ER chaperone, binding immunoglobulin protein (BiP) (34), allowing Sig-1Rs to chaperone inositol 1,4,5-trisphosphate receptors (IP3Rs) at the MAM (34). This enhances Ca2+ signaling from the ER into mitochondria (34, 35), activates the tricarboxylic acid (TCA) cycle, and increases adenosine triphosphate (ATP) production (35). (B) Higher concentrations of DMT cause the translocation of Sig-1Rs from the MAM to the plasma membrane, leading to the inhibition of ion channels. Thus, Sig-1R ligands might shift the site of action of Sig-1R chaperones from the center of the cell to its periphery. In the present scheme, Sig-1Rs and related molecules or organelles are illustrated in the postsynaptic region for the sake of simplicity, although they may also be present presynaptically or in glia.
Similar articles
- The hallucinogen N,N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator.
Fontanilla D, Johannessen M, Hajipour AR, Cozzi NV, Jackson MB, Ruoho AE. Fontanilla D, et al. Science. 2009 Feb 13;323(5916):934-7. doi: 10.1126/science.1166127. Science. 2009. PMID: 19213917 Free PMC article. - N, N-dimethyltryptamine (DMT) in rodent brain: Concentrations, distribution, and recent pharmacological data.
Barker SA. Barker SA. Prog Neuropsychopharmacol Biol Psychiatry. 2025 Mar 20;137:111259. doi: 10.1016/j.pnpbp.2025.111259. Epub 2025 Jan 19. Prog Neuropsychopharmacol Biol Psychiatry. 2025. PMID: 39832749 Review. - N, N-Dimethyltryptamine, a natural hallucinogen, ameliorates Alzheimer's disease by restoring neuronal Sigma-1 receptor-mediated endoplasmic reticulum-mitochondria crosstalk.
Cheng D, Lei ZG, Chu K, Lam OJH, Chiang CY, Zhang ZJ. Cheng D, et al. Alzheimers Res Ther. 2024 May 1;16(1):95. doi: 10.1186/s13195-024-01462-3. Alzheimers Res Ther. 2024. PMID: 38693554 Free PMC article. - A possibly sigma-1 receptor mediated role of dimethyltryptamine in tissue protection, regeneration, and immunity.
Frecska E, Szabo A, Winkelman MJ, Luna LE, McKenna DJ. Frecska E, et al. J Neural Transm (Vienna). 2013 Sep;120(9):1295-303. doi: 10.1007/s00702-013-1024-y. Epub 2013 Apr 26. J Neural Transm (Vienna). 2013. PMID: 23619992 Review. - Significance of mammalian N, N-dimethyltryptamine (DMT): A 60-year-old debate.
Jiménez JH, Bouso JC. Jiménez JH, et al. J Psychopharmacol. 2022 Aug;36(8):905-919. doi: 10.1177/02698811221104054. Epub 2022 Jun 13. J Psychopharmacol. 2022. PMID: 35695604 Review.
Cited by
- A Comprehensive Review of the Current Status of the Cellular Neurobiology of Psychedelics.
Banushi B, Polito V. Banushi B, et al. Biology (Basel). 2023 Oct 28;12(11):1380. doi: 10.3390/biology12111380. Biology (Basel). 2023. PMID: 37997979 Free PMC article. Review. - The Bright Side of Psychedelics: Latest Advances and Challenges in Neuropharmacology.
Mastinu A, Anyanwu M, Carone M, Abate G, Bonini SA, Peron G, Tirelli E, Pucci M, Ribaudo G, Oselladore E, Premoli M, Gianoncelli A, Uberti DL, Memo M. Mastinu A, et al. Int J Mol Sci. 2023 Jan 10;24(2):1329. doi: 10.3390/ijms24021329. Int J Mol Sci. 2023. PMID: 36674849 Free PMC article. Review. - Systems-level view of cocaine addiction: the interconnection of the immune and nervous systems.
Marasco CC, Goodwin CR, Winder DG, Schramm-Sapyta NL, McLean JA, Wikswo JP. Marasco CC, et al. Exp Biol Med (Maywood). 2014 Nov;239(11):1433-42. doi: 10.1177/1535370214537747. Epub 2014 Jun 5. Exp Biol Med (Maywood). 2014. PMID: 24903164 Free PMC article. Review. - The pharmacology of sigma-1 receptors.
Maurice T, Su TP. Maurice T, et al. Pharmacol Ther. 2009 Nov;124(2):195-206. doi: 10.1016/j.pharmthera.2009.07.001. Epub 2009 Jul 18. Pharmacol Ther. 2009. PMID: 19619582 Free PMC article. Review. - Clinical applications of hallucinogens: A review.
Garcia-Romeu A, Kersgaard B, Addy PH. Garcia-Romeu A, et al. Exp Clin Psychopharmacol. 2016 Aug;24(4):229-68. doi: 10.1037/pha0000084. Exp Clin Psychopharmacol. 2016. PMID: 27454674 Free PMC article. Review.
References
- McKenna DJ, Towers GH, Abbott F. Monoamine oxidase inhibitors in South American hallucinogenic plants: Tryptamine and beta-carboline constituents of ayahuasca. J Ethnopharmacol. 1984;10:195–223. - PubMed
- Gambelunghe C, Aroni K, Rossi R, Moretti L, Bacci M. Identification of N,N-dimethyltryptaime and beta-carbolines in psychotropic ayahuasca beverage. Biomed Chromatogr. 2008;22:1056–1059. - PubMed
- Szara I, Sai-Halasz A, Boszormenyi Z. Dimethyltryptamine as a new psychotic agent. Acta Physiol Hung. 1957;11:78–79. - PubMed
- Sai-Halasz A, Brunecker G, Szara S. Dimethyl-tryptamine: A new psychoactive drug. Psychiatr Neurol (Basel) 1958;135:285–301. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources