How ERK1/2 activation controls cell proliferation and cell death: Is subcellular localization the answer? - PubMed (original) (raw)
Review
. 2009 Apr 15;8(8):1168-75.
doi: 10.4161/cc.8.8.8147. Epub 2009 Apr 11.
Affiliations
- PMID: 19282669
- PMCID: PMC2728430
- DOI: 10.4161/cc.8.8.8147
Review
How ERK1/2 activation controls cell proliferation and cell death: Is subcellular localization the answer?
Yohannes Mebratu et al. Cell Cycle. 2009.
Abstract
Extracellular signal-regulated protein kinases 1 and 2 (ERK1/2) are members of the mitogen-activated protein kinase super family that can mediate cell proliferation and apoptosis. The Ras-Raf-MEK-ERK signaling cascade controlling cell proliferation has been well studied but the mechanisms involved in ERK1/2-mediated cell death are largely unknown. This review focuses on recent papers that define ERK1/2 translocation to the nucleus and the proteins involved in the cytosolic retention of activated ERK1/2. Cytosolic retention of ERK1/2 denies access to the transcription factor substrates that are responsible for the mitogenic response. In addition, cytosolic ERK1/2, besides inhibiting survival and proliferative signals in the nucleus, potentiates the catalytic activity of some proapoptotic proteins such as DAP kinase in the cytoplasm. Studies that further define the function of cytosolic ERK1/2 and its cytosolic substrates that enhance cell death will be essential to harness this pathway for developing effective treatments for cancer and chronic inflammatory diseases.
Figures
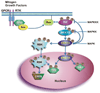
Figure 1. Mechanism of ERK activation and cell proliferation
Activation of receptor tyrosine kinases (RTKs) or G protein-coupled receptors (GPCRs) by growth factors or mitogens leads to the recruitment of an adaptor protein Grb2 (growth factor receptor bound protein) and the guanine nucleotide exchange factor (SOS). The SOS activates Ras to recruit and activate Raf at the plasma membrane by phosphorylation at multiple sites. MEK1/2 is which then phosphorylated at two serine residues that subsequently phosphorylates ERK1/2 on both threonine and tyrosine. Activated ERK1/2 phosphorylates RSK and both RSK and ERK translocate to the nucleus where they activates multiple transcription factors ultimately resulting in effector protein synthesis and causing changes in cell proliferation and survival. ERK phosphorylation of MEK and possibly Raf can inactivate the pathway at those steps creating a negative feedback loop.
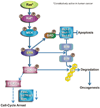
Figure 2. Mechanisms of ERK1/2-mediated oncogenesis
ERK1/2 activation promotes metaplasia and tumor development by phosphorylating Bim and Bid and causing the proteosomal degradation of Bim and the sequestration of Bad to the phosphoserine-binding proteins and, thereby, inhibiting apoptosis. In a separate pathway, ERK1/2 activation phosphorylates FOXO3a at Ser 294, Ser 344, and Ser 425 and facilitates FOXO3a-MDM2 interaction. This interaction enhances FOXO3a degradation through a MDM2-dependent ubiquitin-proteosome pathway, leading to tumor development.
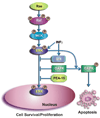
Figure 3. Mechanism of ERK1/2-mediated cell death
The cytoplasmic of ERK1/2 by Bik, PEA-15 or DAPK plays a major role in ERK1/2-mediated cell death. Activated ERK1/2 interacts with PEA-15, Bik, and DAPK and is sequestered in the cytoplasm. Inhibition of ERK1/2 nuclear localization impairs ERK1/2-mediated survival signals and in addition augments the proapoptotic signals of DAPK by phosphorylating the cytoplasmic DAPK.
Similar articles
- Fidelity and spatio-temporal control in MAP kinase (ERKs) signalling.
Pouysségur J, Volmat V, Lenormand P. Pouysségur J, et al. Biochem Pharmacol. 2002 Sep;64(5-6):755-63. doi: 10.1016/s0006-2952(02)01135-8. Biochem Pharmacol. 2002. PMID: 12213567 Review. - Flagellin and lipopolysaccharide stimulate the MEK-ERK signaling pathway in chicken heterophils through differential activation of the small GTPases, Ras and Rap1.
Kogut MH, Genovese KJ, He H. Kogut MH, et al. Mol Immunol. 2007 Mar;44(7):1729-36. doi: 10.1016/j.molimm.2006.07.292. Epub 2006 Oct 12. Mol Immunol. 2007. PMID: 17045653 - ERK1/2 MAP kinases: structure, function, and regulation.
Roskoski R Jr. Roskoski R Jr. Pharmacol Res. 2012 Aug;66(2):105-43. doi: 10.1016/j.phrs.2012.04.005. Epub 2012 Apr 27. Pharmacol Res. 2012. PMID: 22569528 Review. - A cellular threshold for active ERK1/2 levels determines Raf/MEK/ERK-mediated growth arrest versus death responses.
Hong SK, Wu PK, Park JI. Hong SK, et al. Cell Signal. 2018 Jan;42:11-20. doi: 10.1016/j.cellsig.2017.10.001. Epub 2017 Oct 3. Cell Signal. 2018. PMID: 28986121 Free PMC article. - cAMP inhibits the proliferation of retinal pigmented epithelial cells through the inhibition of ERK1/2 in a PKA-independent manner.
Hecquet C, Lefevre G, Valtink M, Engelmann K, Mascarelli F. Hecquet C, et al. Oncogene. 2002 Sep 5;21(39):6101-12. doi: 10.1038/sj.onc.1205765. Oncogene. 2002. PMID: 12203122
Cited by
- Transforming growth factor beta signaling and decidual integrity in mice†.
Fang X, Ni N, Gao Y, Lydon JP, Ivanov I, Rijnkels M, Bayless KJ, Li Q. Fang X, et al. Biol Reprod. 2020 Dec 1;103(6):1186-1198. doi: 10.1093/biolre/ioaa155. Biol Reprod. 2020. PMID: 32902612 Free PMC article. - Effects of prenatal synthetic cannabinoid exposure on the cerebellum of adolescent rat offspring.
Pinky PD, Majrashi M, Fujihashi A, Bloemer J, Govindarajulu M, Ramesh S, Reed MN, Moore T, Suppiramaniam V, Dhanasekaran M. Pinky PD, et al. Heliyon. 2021 Apr 13;7(4):e06730. doi: 10.1016/j.heliyon.2021.e06730. eCollection 2021 Apr. Heliyon. 2021. PMID: 33912711 Free PMC article. - Cytoplasmic BRMS1 expression in malignant melanoma is associated with increased disease-free survival.
Slipicevic A, Holm R, Emilsen E, Ree Rosnes AK, Welch DR, Mælandsmo GM, Flørenes VA. Slipicevic A, et al. BMC Cancer. 2012 Feb 22;12:73. doi: 10.1186/1471-2407-12-73. BMC Cancer. 2012. PMID: 22356677 Free PMC article. - Upregulated Ras/Raf/ERK1/2 signaling pathway: a new hope in the repair of spinal cord injury.
Liu T, Cao FJ, Xu DD, Xu YQ, Feng SQ. Liu T, et al. Neural Regen Res. 2015 May;10(5):792-6. doi: 10.4103/1673-5374.156984. Neural Regen Res. 2015. PMID: 26109956 Free PMC article. - Endogenous repair by the activation of cell survival signalling cascades during the early stages of rat Parkinsonism.
Lui NP, Chen LW, Yung WH, Chan YS, Yung KK. Lui NP, et al. PLoS One. 2012;7(12):e51294. doi: 10.1371/journal.pone.0051294. Epub 2012 Dec 12. PLoS One. 2012. PMID: 23251488 Free PMC article.
References
- Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–3310. - PubMed
- Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. - PubMed
- Omori S, Hida M, Fujita H, Takahashi H, Tanimura S, Kohno M, Awazu M. Extracellular signal-regulated kinase inhibition slows disease progression in mice with polycystic kidney disease. J Am Soc Nephrol. 2006;17:1604–1614. - PubMed
- Ravingerova T, Barancik M, Strniskova M. Mitogen-activated protein kinases: a new therapeutic target in cardiac pathology. Mol Cell Biochem. 2003;247:127–138. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL068111-06/HL/NHLBI NIH HHS/United States
- R56 HL068111/HL/NHLBI NIH HHS/United States
- HL 68111/HL/NHLBI NIH HHS/United States
- R01 HL068111/HL/NHLBI NIH HHS/United States
- R01 ES015482/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous