Sympathetic nervous system control of anti-influenza CD8+ T cell responses - PubMed (original) (raw)
Sympathetic nervous system control of anti-influenza CD8+ T cell responses
Kristie M Grebe et al. Proc Natl Acad Sci U S A. 2009.
Abstract
Despite the longstanding appreciation of communication between the nervous and the immune systems, the nature and significance of these interactions to immunity remain enigmatic. Here, we show that 6-hydroxydopamine-mediated ablation of the mouse peripheral sympathetic nervous system increases primary CD8(+) T cell responses to viral and cellular antigens presented by direct priming or cross-priming. The sympathetic nervous system also suppresses antiviral CD4(+) T cell responses, but this is not required for suppressing CD8(+) T cell responses. Adoptive transfer experiments indicate that enhanced CD8(+) responses do not result from permanent alterations in CD8(+) T cell function in sympathectomized mice. Rather, additional findings suggest that the sympathetic nervous system tempers the capacity of antigen-presenting cells to activate naïve CD8(+) T cells. We also show that antiviral CD8(+) T cell responses are enhanced by administration of a beta(2) (but not beta(1) or alpha) adrenergic antagonist. These findings demonstrate a critical role for the sympathetic nervous system in limiting CD8(+) T cell responses and indicate that CD8(+) T cell responses may be altered in patients using beta-blockers, one of the most widely prescribed classes of drugs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
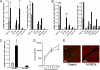
Fig. 1.
IAV-specific CD8+ T cell responses but not antibody responses are increased in 6-OHDA-treated mice. Black bars represent 6-OHDA-treated mice, and gray bars represent saline-treated mice. CD8+ T cell responses were measured against PR8-infected cells (PR8 EL4) and viral peptides [NP 366, PA 224, NS2 114, and PB1(F2) 62]. Uninfected EL4 and OVA 257 peptides were used as controls. Experiments were performed independently 3 times for each assay. *, P < 0.05; **, P < 0.005. (A) Mice were infected i.p. with PR8, and anti-IAV TCD8+ responses were measured on day 7 after infection. Spleens were analyzed individually from 3 mice per group. PECs were pooled from 3 mice per group for analysis. (B) Mice were infected i.n. with PR8 (0.1 LD50). Ten days after infection, spleens were harvested and analyzed for flu-specific TCD8+. Spleens were analyzed individually from 3 mice per group. The T cell response in the BAL was measured on day 7 after infection, and BAL fluid was pooled from 5 mice per group. (C) Mice were infected i.p. with PR8, and the anti-IAV TCD4+ response to UV-inactivated PR8 was measured 7 days after infection. Spleens were analyzed individually from 3 mice per group. (D) Mice were bled before and at days 7, 14, and 21 after infection i.p. with PR8, and antibody titers were determined by ELISA. Solid line with triangles represents control mice, and dashed line with squares represents 6-OHDA-treated mice. (E) Spleens from control or 6-OHDA-treated mice were harvested 1 week after infection with PR8 i.p. and were analyzed for sympathectomy by SPG fluorescence. Representative pictures of blood vessels (seen as dark areas) from each group are shown. Green staining represents the sympathetic nerves, and the red stain is malachite green counterstain to view the surrounding tissue.
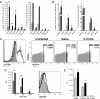
Fig. 2.
6-OHDA treatment enhances CD8+ T cell responses to both direct and crossprimed antigens by enhancing the stimulatory capacity of the pAPC. Black bars represent 6-OHDA-treated mice, and gray bars represent saline-treated mice. *, P < 0.05; **, P < 0.005; ***, P < 0.0005. (A) Mice challenged i.p. with KD2SV cells. Seven days later, SV40 CD8+ T cell responses against SV40 (Tag 206, Tag 404, Tag 289, and Tag 223) and control (OVA 257) peptides and C57SV cells were measured. Responses in the spleen were analyzed from 3 individual mice, and PECs were pooled from 3 mice per group. Experiments were performed independently 3 times. (B) Mice were infected i.p. with recombinant VV-VFP-Ub-OVA257 minigene, and the CD8+ T cell responses against VV (B8R 20), Minigene (OVA 257), and control (NP366) peptides were measured. Responses were analyzed from 3 individual mice per group for the spleen and PEC. Experiments were performed independently 3 times. (C) Mice were infected i.v. with VV-encoding minigenes, and spleens were stained with 25D-1.16. Solid gray represents uninfected mice, dotted line represents mice infected with irrelevant virus (VV-VFP-Ub-NP366), solid line represents control mice infected with VV-VFP-Ub-OVA257, and the dashed line represents 6-OHDA-treated mice infected with VV-VFP-Ub-OVA257. The number of Venus-positive cells is inside the gate, and the median fluorescence intensity (MFI) of the gated population is shown above the gate. This experiment was repeated independently 2 times. (D) pAPC preparations from infected (control: gray bars; 6-OHDA: black bars) and uninfected (control: white bars; 6-OHDA: hatched bars) mice were used to stimulate OT-I T cells ex vivo in a proliferation assay. The pAPC populations were stained with 25D-1.16. Solid gray represents uninfected control mice, dotted line represents uninfected 6-OHDA-treated mice, solid line represents control mice infected with PR8-OVA, and the dashed line represents 6-OHDA-treated mice infected with PR8-OVA. Spleens from 3 mice per group were pooled to generate pAPC preparations. (E) Sorted DC populations from infected mice were used as stimulators in proliferation assay similar to D and plated at a 1:1 ratio with OT-I cells. Spleens from 5 mice per group were pooled to generate the DC populations. Experiments in D and E were repeated independently 3 times.
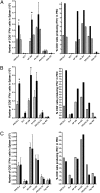
Fig. 3.
Blocking β-ARs but not α-ARs enhances anti-IAV CD8+ T cell responses. (A) Mice were treated with the β-adrenergic blocker nadolol (black bars), the α-blocker phentolamine (hatched bars), or saline (gray bars). Mice were infected i.p. with PR8, and anti-IAV TCD8+ responses were measured on day 7 after infection. TCD8+ responses were measured against PR8-infected cells (PR8 EL4) and viral peptides [NP 366, PA 224, and PB1(F2) 62]. Uninfected EL4 and OVA 257 peptides were used as controls. Spleens were analyzed individually from 3 mice per group. PEC samples were pooled from 3 mice per group for analysis. The experiment was performed independently 3 times. (B) Mice were treated with the β2-adrenergic blocker ICI118,551 (black bars) or vehicle (gray bars), and TCD8+ response was measured as in A. (C) Mice were treated with the β1-adrenergic blocker metoprolol (black bars) or vehicle (gray bars), and TCD8+ response was measured as in A. Experiments in both B and C were repeated independently 3 times. *, P < 0.05.
Similar articles
- Lung CD103+ dendritic cells efficiently transport influenza virus to the lymph node and load viral antigen onto MHC class I for presentation to CD8 T cells.
Ho AW, Prabhu N, Betts RJ, Ge MQ, Dai X, Hutchinson PE, Lew FC, Wong KL, Hanson BJ, Macary PA, Kemeny DM. Ho AW, et al. J Immunol. 2011 Dec 1;187(11):6011-21. doi: 10.4049/jimmunol.1100987. Epub 2011 Oct 31. J Immunol. 2011. PMID: 22043017 - Acute Traumatic Brain Injury Induces CD4+ and CD8+ T Cell Functional Impairment by Upregulating the Expression of PD-1 via the Activated Sympathetic Nervous System.
Yang Y, Ye Y, Chen C, Kong C, Su X, Zhang X, Bai W, He X. Yang Y, et al. Neuroimmunomodulation. 2019;26(1):43-57. doi: 10.1159/000495465. Epub 2019 Jan 29. Neuroimmunomodulation. 2019. PMID: 30695785 - PTPN2 restrains CD8⁺ T cell responses after antigen cross-presentation for the maintenance of peripheral tolerance in mice.
Wiede F, Ziegler A, Zehn D, Tiganis T. Wiede F, et al. J Autoimmun. 2014 Sep;53:105-14. doi: 10.1016/j.jaut.2014.05.008. Epub 2014 Jul 2. J Autoimmun. 2014. PMID: 24997008 - CD8+ T-cell priming regulated by cytokines of the innate immune system.
Stäger S, Kaye PM. Stäger S, et al. Trends Mol Med. 2004 Aug;10(8):366-71. doi: 10.1016/j.molmed.2004.06.003. Trends Mol Med. 2004. PMID: 15310456 Review. - Cross-presentation: inducing CD8 T cell immunity and tolerance.
Kurts C. Kurts C. J Mol Med (Berl). 2000;78(6):326-32. doi: 10.1007/s001090000108. J Mol Med (Berl). 2000. PMID: 11001529 Review.
Cited by
- Loss of direct adrenergic innervation after peripheral nerve injury causes lymph node expansion through IFN-γ.
Chen CS, Weber J, Holtkamp SJ, Ince LM, de Juan A, Wang C, Lutes L, Barnoud C, Kizil B, Hergenhan SM, Salvermoser J, Lasch M, Deindl E, Schraml B, Baumjohann D, Scheiermann C. Chen CS, et al. J Exp Med. 2021 Aug 2;218(8):e20202377. doi: 10.1084/jem.20202377. Epub 2021 Jun 4. J Exp Med. 2021. PMID: 34086056 Free PMC article. - β-Adrenergic Signaling in Mice Housed at Standard Temperatures Suppresses an Effector Phenotype in CD8+ T Cells and Undermines Checkpoint Inhibitor Therapy.
Bucsek MJ, Qiao G, MacDonald CR, Giridharan T, Evans L, Niedzwecki B, Liu H, Kokolus KM, Eng JW, Messmer MN, Attwood K, Abrams SI, Hylander BL, Repasky EA. Bucsek MJ, et al. Cancer Res. 2017 Oct 15;77(20):5639-5651. doi: 10.1158/0008-5472.CAN-17-0546. Epub 2017 Aug 17. Cancer Res. 2017. PMID: 28819022 Free PMC article. - Stress-induced glucocorticoids at the earliest stages of herpes simplex virus-1 infection suppress subsequent antiviral immunity, implicating impaired dendritic cell function.
Elftman MD, Hunzeker JT, Mellinger JC, Bonneau RH, Norbury CC, Truckenmiller ME. Elftman MD, et al. J Immunol. 2010 Feb 15;184(4):1867-75. doi: 10.4049/jimmunol.0902469. Epub 2010 Jan 20. J Immunol. 2010. PMID: 20089700 Free PMC article. - Adrenergic signaling controls early transcriptional programs during CD8+ T cell responses to viral infection.
Estrada LD, Ağaç Çobanoğlu D, Wise A, Maples RW, Çobanoğlu MC, Farrar JD. Estrada LD, et al. PLoS One. 2022 Aug 9;17(8):e0272017. doi: 10.1371/journal.pone.0272017. eCollection 2022. PLoS One. 2022. PMID: 35944008 Free PMC article. - Neuroscience in peripheral cancers: tumors hijacking nerves and neuroimmune crosstalk.
Fan HY, Liang XH, Tang YL. Fan HY, et al. MedComm (2020). 2024 Oct 31;5(11):e784. doi: 10.1002/mco2.784. eCollection 2024 Nov. MedComm (2020). 2024. PMID: 39492832 Free PMC article. Review.
References
- Ader R, Felten D, Cohen N. Interactions between the brain and the immune system. Annu Rev Pharmacol Toxicol. 1990;30:561–602. - PubMed
- Calvo W. The innervation of the bone marrow in laboratory animals. Am J Anat. 1968;123:315–328. - PubMed
- Reilly FD, McCuskey PA, Miller ML, McCuskey RS, Meineke HA. Innervation of the periarteriolar lymphatic sheath of the spleen. Tissue Cell. 1979;11:121–126. - PubMed
- Williams JM, Felten DL. Sympathetic innervation of murine thymus and spleen: A comparative histofluorescence study. Anat Rec. 1981;199:531–542. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials