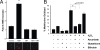Bilirubin and glutathione have complementary antioxidant and cytoprotective roles - PubMed (original) (raw)
Bilirubin and glutathione have complementary antioxidant and cytoprotective roles
Thomas W Sedlak et al. Proc Natl Acad Sci U S A. 2009.
Abstract
Glutathione (GSH) and bilirubin are prominent endogenous antioxidant cytoprotectants. Despite tissue levels that are thousands of times lower than GSH, bilirubin is effective because of the biosynthetic cycle wherein it is generated from biliverdin by biliverdin reductase (BVR). When bilirubin acts as an antioxidant, it is oxidized to biliverdin, which is immediately reduced by BVR to bilirubin. Why does the body employ both of these 2 distinct antioxidant systems? We show that the water-soluble GSH primarily protects water soluble proteins, whereas the lipophilic bilirubin protects lipids from oxidation. Mice with deletion of heme oxygenase-2, which generates biliverdin, display greater lipid than protein oxidation, while the reverse holds for GSH depletion. RNA interference depletion of BVR increases oxidation of lipids more than protein. Depletion of BVR or GSH augments cell death in an oxidant-specific fashion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Increased lipid but not protein oxidation in heme oxygenase-2 knockout (HO2 −/−) animals. (A) Increased lipid oxidation product 4-hydroxynonenal (4-HNE) in HO2 (−/−) hippocampus. Data represent mean ± SEM from 4 samples. *, P < 0.001 by t test. (B and C) Similar levels of protein carbonyls, a measure of protein oxidation, in wild-type and HO2 (−/−) animals. Liver (B) and brain (C) were homogenized from 6-month-old male wild-type and HO2 (−/−) animals and protein carbonyls were determined by derivatization with 2,4-dinitrophenylhydrazine (DNPH). To verify measurement of increased carbonyls, samples were treated 30 min with 1 mM tert-butyl hydroperoxide (TBH). Means ± SEM are shown for 4 animals from 2 independent studies.
Fig. 2.
Differential protection of lipid and protein oxidation by bilirubin and glutathione. (A) Bilirubin blocks oxidant-induced accumulation of 4-hydroxynonenal (4-HNE) adducts. HEK293 cells were treated with combinations of 500 μM tert-butyl hydroperoxide (TBH) and 100 nM bilirubin (BR) for 16 h and stained for 4-HNE. Data represent means ± SEM from 4 samples. *, P < 0.001 from each other treatment (ANOVA followed by Tukey-Kramer post hoc test). Representative of 3 experiments. (B) Greater potency of water-soluble antioxidants for protein oxidation than bilirubin. HEK293 cells were radiolabeled with [35S]methionine, and protein was oxidized with combinations of H2O2 (10 μM), bilirubin (5 μM), and the water-soluble antioxidants, glutathione (5 μM) and ascorbate (5 μM). Data represent means ± SEM of triplicate determinations, representative of 3 experiments. *, P < 0.001 (ANOVA followed by Tukey-Kramer post hoc test).
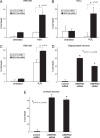
Fig. 3.
Depletion of BVR preferentially increases lipid oxidation while GSH depletion increases protein oxidation. (A) siRNA depletion of biliverdin reductase (BVR). HEK293 cells were transfected with the indicated siRNA and lysates immunoblotted at 72 h. siRNA 180 and 728 had the greatest efficacy in depleting BVR. Representative of 3 experiments. (B and C) Greater lipid oxidation follows depletion of biliverdin reductase (BVR) than glutathione. HEK293 cells were transfected with BVR siRNA (180) or control siRNA (muBVR), and then treated with 1 mM buthionine sulfoximine (BSO) at 48 h to rapidly deplete glutathione. At 72 h posttransfection, cells were treated with 250 μM H2O2 for 16 h and stained for lipid oxidation product, 4-hydroxynonenal (4-HNE). In C, protein carbonyls were quantified by derivatization with 2,4-dinitrophenylhydrazine (DNPH). Data represent means ± SEM of quadruplicate determinations. *, P < 0.01 (ANOVA followed by Tukey-Kramer post hoc test) (B); *, P < 0.05 (t test) (C).
Fig. 4.
Increased oxidant-induced cell death following depletion of BVR. (A–C) Increased susceptibility to oxidant-induced death in HEK293 and HeLa cells depleted of BVR by 180 and 728 siRNA. HEK293 cells were treated with the indicated siRNA for 72 h then exposed to hydrogen peroxide (H2O2) or tert-butyl hydroperoxide (TBH) for 18 h. Cell death was assayed by MTT assay. Both the 180 and 728 siRNA elicited increased oxidant-induced cell death. (A) HEK 293 cells treated with 728 siRNA and TBH. (B) HeLa cells treated with 180 siRNA and H2O2. (C) HEK 293 cells treated with 180 siRNA and H2O2. Data are the means of triplicates ± SEM, representative of 2 experiments for each. *, P < 0.05 by t test. Control oligonucleotides: (A and B) muBVR, (C) BVR180c. (D and E) Neuroprotective effect of BVR in primary hippocampal (D) and cortical (E) neurons. Two different siRNAs targeting endogenous rat BVR (ratBVR1 and ratBVR2) or control siRNA directed against mouse BVR were cotransfected with eGFP. At 72 h posttransfection, surviving GFP positive neurons were blindly counted under 20× magnification in 10 fields. Data are representative of 4 independent transfections. *, P < 0.01 vs. control (ANOVA followed by Tukey-Kramer post hoc test).
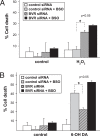
Fig. 5.
BVR and glutathione (GSH) pathways, respectively, protect against oxidants that differentially target lipids and protein. (A) Depletion of BVR enhances hydrogen peroxide (H2O2)-induced cell death greater than glutathione depletion. HEK293 cells were transfected with the BVR or control siRNA, then treated with 1 mM BSO at 48 h to deplete glutathione. At 72 h posttransfection, cells were treated for 14 h with 500 μM H2O2 and cell death was determined by MTT assay. BVR depleted cells exhibit markedly increased cell death compared to GSH-depleted cells. (B) Greater protection against 6-hydroxydopamine (6-OH DA)-induced cell death is provided by the GSH pathway than the bilirubin pathway. Cells were depleted of BVR and GSH as in A, then treated 14 h with 300 μM 6-OH DA. Cell death is greater following depletion of GSH than BVR. Data are means of triplicates from at least 3 independent experiments. *, P < 0.05 by t test.
Similar articles
- Limited role for the bilirubin-biliverdin redox amplification cycle in the cellular antioxidant protection by biliverdin reductase.
Maghzal GJ, Leck MC, Collinson E, Li C, Stocker R. Maghzal GJ, et al. J Biol Chem. 2009 Oct 23;284(43):29251-9. doi: 10.1074/jbc.M109.037119. Epub 2009 Aug 18. J Biol Chem. 2009. PMID: 19690164 Free PMC article. - Biliverdin reductase: a major physiologic cytoprotectant.
Baranano DE, Rao M, Ferris CD, Snyder SH. Baranano DE, et al. Proc Natl Acad Sci U S A. 2002 Dec 10;99(25):16093-8. doi: 10.1073/pnas.252626999. Epub 2002 Nov 27. Proc Natl Acad Sci U S A. 2002. PMID: 12456881 Free PMC article. - Conversion of biliverdin to bilirubin by biliverdin reductase contributes to endothelial cell protection by heme oxygenase-1-evidence for direct and indirect antioxidant actions of bilirubin.
Jansen T, Hortmann M, Oelze M, Opitz B, Steven S, Schell R, Knorr M, Karbach S, Schuhmacher S, Wenzel P, Münzel T, Daiber A. Jansen T, et al. J Mol Cell Cardiol. 2010 Aug;49(2):186-95. doi: 10.1016/j.yjmcc.2010.04.011. Epub 2010 Apr 27. J Mol Cell Cardiol. 2010. PMID: 20430037 - Bilirubin benefits: cellular protection by a biliverdin reductase antioxidant cycle.
Sedlak TW, Snyder SH. Sedlak TW, et al. Pediatrics. 2004 Jun;113(6):1776-82. doi: 10.1542/peds.113.6.1776. Pediatrics. 2004. PMID: 15173506 Review. No abstract available. - Biliverdin reductase: new features of an old enzyme and its potential therapeutic significance.
Florczyk UM, Jozkowicz A, Dulak J. Florczyk UM, et al. Pharmacol Rep. 2008 Jan-Feb;60(1):38-48. Pharmacol Rep. 2008. PMID: 18276984 Free PMC article. Review.
Cited by
- The human biliverdin reductase-based peptide fragments and biliverdin regulate protein kinase Cδ activity: the peptides are inhibitors or substrate for the protein kinase C.
Miralem T, Lerner-Marmarosh N, Gibbs PE, Tudor C, Hagen FK, Maines MD. Miralem T, et al. J Biol Chem. 2012 Jul 13;287(29):24698-712. doi: 10.1074/jbc.M111.326504. Epub 2012 May 14. J Biol Chem. 2012. PMID: 22584576 Free PMC article. - Evolution of inflammatory diseases.
Okin D, Medzhitov R. Okin D, et al. Curr Biol. 2012 Sep 11;22(17):R733-40. doi: 10.1016/j.cub.2012.07.029. Curr Biol. 2012. PMID: 22975004 Free PMC article. Review. - Effect of repeatedly heated palm olein on blood pressure-regulating enzymes activity and lipid peroxidation in rats.
Leong XF, Salimon J, Mustafa MR, Jaarin K. Leong XF, et al. Malays J Med Sci. 2012 Jan;19(1):20-9. Malays J Med Sci. 2012. PMID: 22977371 Free PMC article. - Total bilirubin is negatively related to diabetes mellitus in Chinese elderly: a community study.
Zhong P, Sun D, Wu D, Liu X. Zhong P, et al. Ann Transl Med. 2019 Sep;7(18):474. doi: 10.21037/atm.2019.07.104. Ann Transl Med. 2019. PMID: 31700910 Free PMC article. - Hyaluronic acid-bilirubin nanomedicine for targeted modulation of dysregulated intestinal barrier, microbiome and immune responses in colitis.
Lee Y, Sugihara K, Gillilland MG 3rd, Jon S, Kamada N, Moon JJ. Lee Y, et al. Nat Mater. 2020 Jan;19(1):118-126. doi: 10.1038/s41563-019-0462-9. Epub 2019 Aug 19. Nat Mater. 2020. PMID: 31427744 Free PMC article.
References
- Maines MD. The heme oxygenase system. Antioxid Redox Signal. 2005;7:1761–1766. - PubMed
- Verma A, Hirsch DJ, Glatt CE, Ronnett GV, Snyder SH. Carbon monoxide: a putative neural messenger. Science. 1993;259:381–384. - PubMed
- Boehning D, Sedaghat L, Sedlak TW, Snyder SH. Heme oxygenase-2 is activated by calcium-calmodulin. J Biol Chem. 2004;279:30927–30930. - PubMed
- Kutty RK, Maines MD. Purification and characterization of biliverdin reductase from rat liver. J Biol Chem. 1981;256:3956–3962. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DA00266/DA/NIDA NIH HHS/United States
- K05 DA000074-29/DA/NIDA NIH HHS/United States
- P50 DA000266-37/DA/NIDA NIH HHS/United States
- DA00074/DA/NIDA NIH HHS/United States
- K08 NS057824/NS/NINDS NIH HHS/United States
- 1K08NS057824/NS/NINDS NIH HHS/United States
- P50 DA000266/DA/NIDA NIH HHS/United States
- K05 DA000074/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases