JAK2 haplotype is a major risk factor for the development of myeloproliferative neoplasms - PubMed (original) (raw)
doi: 10.1038/ng.334. Epub 2009 Mar 15.
Andrew Chase, Richard T Silver, David Oscier, Katerina Zoi, Y Lynn Wang, Holger Cario, Heike L Pahl, Andrew Collins, Andreas Reiter, Francis Grand, Nicholas C P Cross
Affiliations
- PMID: 19287382
- PMCID: PMC4120192
- DOI: 10.1038/ng.334
JAK2 haplotype is a major risk factor for the development of myeloproliferative neoplasms
Amy V Jones et al. Nat Genet. 2009 Apr.
Abstract
Chronic myeloproliferative neoplasms (MPNs) are a group of related conditions characterized by the overproduction of cells from one or more myeloid lineages. More than 95% of cases of polycythemia vera, and roughly half of essential thrombocythemia and primary myelofibrosis acquire a unique somatic 1849G>T JAK2 mutation (encoding V617F) that is believed to be a critical driver of excess proliferation. We report here that JAK2(V617F)-associated disease is strongly associated with a specific constitutional JAK2 haplotype, designated 46/1, in all three disease entities compared to healthy controls (polycythemia vera, n = 192, P = 2.9 x 10(-16); essential thrombocythemia, n = 78, P = 8.2 x 10(-9) and myelofibrosis, n = 41, P = 8.0 x 10(-5)). Furthermore, JAK2(V617F) specifically arises on the 46/1 allele in most cases. The 46/1 JAK2 haplotype thus predisposes to the development of JAK2(V617F)-associated MPNs (OR = 3.7; 95% CI = 3.1-4.3) and provides a model whereby a constitutional genetic factor is associated with an increased risk of acquiring a specific somatic mutation.
Figures
Figure 1
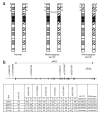
Allele distortions due to aUPD enable direct reading of JAK2 haplotypes. (a) The _JAK2_V617F mutation (indicated by an asterisk) and flanking SNPs are reduced to homozygosity in a proportion of cells following mitotic recombination. (b) SNPs and _JAK2_V617F were quantified by pyrosequencing. In many cases that harbored a homozygous _JAK2_V617F clone, it was possible to directly read the haplotype on which the mutation arose by the finding that one allele at each SNP predominated (allelic ratio ≥0.6, for example, cases E659, E2433 and E2513). In cases with a homozygous clone and %V617F <90%, it was usually possible to read the residual haplotype (that is, the haplotype of the chromosome that had not acquired _JAK2_V617F) by the finding of allelic ratios between 0.1–0.4 (for example, cases E2433 and E2513). Where the homozygous clone was small or nonexistent (most cases with %V617F <60%), neither the _JAK2_V617F nor wild-type haplotype could be read (for example, case E1186).
Figure 2
SNPs, haplotypes and LD around JAK2. (a) The nine most common JAK2 haplotypes in the UK population. The 14 SNPs in bold were analyzed by the WTCCC in 1,500 blood donors from which the frequencies were determined; asterisks indicate SNPs that tag 46/1, which are highlighted in gray. rs78644782, rs10124001 and rs10758669 are immediately upstream of JAK2; all other SNPs are within JAK2 introns. (b) LD in the JAK2 region (HapMap data release 23a/phase II March 2008).
Figure 3
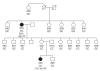
Familial polycythemia vera pedigree. The two affected individuals (UPNs 534 and 533) are shown as black circles. The genotype for rs12340895 is shown (G = 46/1 allele; C = non-46/1 allele), as is the %V617F in affected cases. Allele-specific PCR for UPN 534 showed that _JAK2_V617F arose on the 46/1 allele. All other individuals had normal blood counts and were negative for _JAK2_V617F, PRV1 overexpression and endogenous erythroid colony growth.
Figure 4
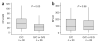
Association between JAK2 haplotype and numbers of hemopoietic colonies. CFU-GM and BFU-E colony growth per 4 × 105 peripheral blood mononuclear cells from 56 healthy controls that were 46/1 nullizygous (C/C at rs12340895, n = 30) or had at least one 46/1 allele (G/C, n = 21; or G/G, n = 5). Box plots illustrate the 95% range (vertical lines), median (horizontal lines) and interquartile range (boxes). Colony numbers were compared by the Mann-Whitney U test.
Comment in
- Somatic and germline genetics at the JAK2 locus.
Campbell PJ. Campbell PJ. Nat Genet. 2009 Apr;41(4):385-6. doi: 10.1038/ng0409-385. Nat Genet. 2009. PMID: 19338077
Similar articles
- A common JAK2 haplotype confers susceptibility to myeloproliferative neoplasms.
Olcaydu D, Harutyunyan A, Jäger R, Berg T, Gisslinger B, Pabinger I, Gisslinger H, Kralovics R. Olcaydu D, et al. Nat Genet. 2009 Apr;41(4):450-4. doi: 10.1038/ng.341. Epub 2009 Mar 15. Nat Genet. 2009. PMID: 19287385 - The G allele of the JAK2 rs10974944 SNP, part of JAK2 46/1 haplotype, is strongly associated with JAK2 V617F-positive myeloproliferative neoplasms.
Trifa AP, Cucuianu A, Petrov L, Urian L, Militaru MS, Dima D, Pop IV, Popp RA. Trifa AP, et al. Ann Hematol. 2010 Oct;89(10):979-83. doi: 10.1007/s00277-010-0960-y. Epub 2010 Apr 27. Ann Hematol. 2010. PMID: 20422415 - A germline JAK2 SNP is associated with predisposition to the development of JAK2(V617F)-positive myeloproliferative neoplasms.
Kilpivaara O, Mukherjee S, Schram AM, Wadleigh M, Mullally A, Ebert BL, Bass A, Marubayashi S, Heguy A, Garcia-Manero G, Kantarjian H, Offit K, Stone RM, Gilliland DG, Klein RJ, Levine RL. Kilpivaara O, et al. Nat Genet. 2009 Apr;41(4):455-9. doi: 10.1038/ng.342. Epub 2009 Mar 15. Nat Genet. 2009. PMID: 19287384 Free PMC article. - JAK2 V617F and beyond: role of genetics and aberrant signaling in the pathogenesis of myeloproliferative neoplasms.
Oh ST, Gotlib J. Oh ST, et al. Expert Rev Hematol. 2010 Jun;3(3):323-37. doi: 10.1586/ehm.10.28. Expert Rev Hematol. 2010. PMID: 21082983 Review.
Cited by
- Second Cancer Onset in Myeloproliferative Neoplasms: What, When, Why?
Cumbo C, Anelli L, Zagaria A, Coccaro N, Tarantini F, Specchia G, Musto P, Albano F. Cumbo C, et al. Int J Mol Sci. 2022 Mar 15;23(6):3177. doi: 10.3390/ijms23063177. Int J Mol Sci. 2022. PMID: 35328597 Free PMC article. Review. - Somatic and germline genetics at the JAK2 locus.
Campbell PJ. Campbell PJ. Nat Genet. 2009 Apr;41(4):385-6. doi: 10.1038/ng0409-385. Nat Genet. 2009. PMID: 19338077 - Recurrent somatic JAK-STAT pathway variants within a RUNX1-mutated pedigree.
Tawana K, Wang J, Király PA, Kállay K, Benyó G, Zombori M, Csomor J, Al Seraihi A, Rio-Machin A, Matolcsy A, Chelala C, Cavenagh J, Fitzgibbon J, Bödör C. Tawana K, et al. Eur J Hum Genet. 2017 Aug;25(8):1020-1024. doi: 10.1038/ejhg.2017.80. Epub 2017 May 17. Eur J Hum Genet. 2017. PMID: 28513614 Free PMC article. - Heritable polymorphism predisposes to high BAALC expression in acute myeloid leukemia.
Eisfeld AK, Marcucci G, Liyanarachchi S, Döhner K, Schwind S, Maharry K, Leffel B, Döhner H, Radmacher MD, Bloomfield CD, Tanner SM, de la Chapelle A. Eisfeld AK, et al. Proc Natl Acad Sci U S A. 2012 Apr 24;109(17):6668-73. doi: 10.1073/pnas.1203756109. Epub 2012 Apr 9. Proc Natl Acad Sci U S A. 2012. PMID: 22493267 Free PMC article. - Cytokine Profiling in Myeloproliferative Neoplasms: Overview on Phenotype Correlation, Outcome Prediction, and Role of Genetic Variants.
Masselli E, Pozzi G, Gobbi G, Merighi S, Gessi S, Vitale M, Carubbi C. Masselli E, et al. Cells. 2020 Sep 21;9(9):2136. doi: 10.3390/cells9092136. Cells. 2020. PMID: 32967342 Free PMC article. Review.
References
- James C, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–1148. - PubMed
- Kralovics R, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N. Engl. J. Med. 2005;352:1779–1790. - PubMed
- Levine RL, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–397. - PubMed
- Baxter EJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–1061. - PubMed
- Kralovics R, Guan Y, Prchal JT. Acquired uniparental disomy of chromosome 9p is a frequent stem cell defect in polycythemia vera. Exp. Hematol. 2002;30:229–236. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous