Cadherin adhesion, tissue tension, and noncanonical Wnt signaling regulate fibronectin matrix organization - PubMed (original) (raw)
Cadherin adhesion, tissue tension, and noncanonical Wnt signaling regulate fibronectin matrix organization
Bette J Dzamba et al. Dev Cell. 2009 Mar.
Abstract
In this study we demonstrate that planar cell polarity signaling regulates morphogenesis in Xenopus embryos in part through the assembly of the fibronectin (FN) matrix. We outline a regulatory pathway that includes cadherin adhesion and signaling through Rac and Pak, culminating in actin reorganization, myosin contractility, and tissue tension, which, in turn, directs the correct spatiotemporal localization of FN into a fibrillar matrix. Increased mechanical tension promotes FN fibril assembly in the blastocoel roof (BCR), while reduced BCR tension inhibits matrix assembly. These data support a model for matrix assembly in tissues where cell-cell adhesions play an analogous role to the focal adhesions of cultured cells by transferring to integrins the tension required to direct FN fibril formation at cell surfaces.
Figures
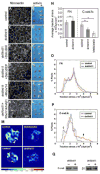
Figure 1. Non-canonical Wnt signaling regulates FN assembly and cadherin adhesion
(A,C,E,G,I, K) FN fibril assembly in dorsal marginal zones from stage 11 embryos was analyzed by immunofluoresence microscopy using anti-FN pAb 32. (A) FN forms a network of fibrils in control dorsal marginal zones by this stage. (C) dnWnt11 inhibits fibril assembly but FN is deposited at cell boundaries. (E) FN fibril assembly is rescued by Dsh lacking the DIX domain. (G) Dsh lacking the DEP domain is unable to rescue fibril assembly. (I) Constitutively active RhoV14 also rescues FN assembly. (K) dn integrin HAβ1 blocks RhoV14 rescue of fibril assembly. Scale bar in K=50 μm. (B,D,F,H,J,L) Animal cap extension assays plus (+) or minus (-) activin. (B) Control activin-treated animal caps extend. (D) Caps expressing dnWnt11 do not extend. (F) Dsh construct lacking the DIX domain rescues cap extension. (H) Dsh lacking the DEP domain does not rescue cap extension. (J) Constitutively active RhoV14 rescues extension. (L) RhoV14 is unable to rescue extension when co-expressed with HAβ1. (M) Representative traction stress maps for BCR cells plated on substrates coated with C-Cad.fc. Colors express the magnitude of traction stress (× 10 4 dyn/cm2) mapped to cell regions. (N) Average traction stress per cell (+/- S.D.) for control, blebbistatin treated and dnWnt11 expressing cells. (*) p < 0.001. (ns) not significant. (O-P) Distribution of traction stress for control and dnWnt11 expressing cells on (O) FN and (P) C-cad.fc substrates. Vertical axis values represent percentage of total pixels with a specific stress value. For panels (N-P) averages were calculated from 10-15 individual cell measurements. Frequencies of low (open arrow) and high (closed arrow) stress attributed to dnWnt11 and control cells, respectively, are indicated. (Q) Western blot to compare cell surface levels of C-cad and β1 integrins in intact biotinylated BCRs, (+) or (-) dnWnt 11.
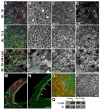
Figure 2. Cadherin adhesion regulates FN fibril assembly
Confocal projections (15 μM) of BCRs from embryos fixed at (A-D) early (st. 10) or (E-H) late (st. 11.5) gastrula stage. BCRs were stained with antibodies against FN (green), C-cad (blue), and phalloidin to visualize filamentous actin (red). (A,E,I) Composite of all 3 fluorochromes. At stage 10, C-cad is noted at cell edges (B) and in linear arrays perpendicular to the cell edge (B, arrows), little cortical actin is present (C) and FN is arranged in punctae at cell surfaces (D). Cells in the BCR are rounded at stage 10. By stage 11, cells are polygonal in shape (compare B and F), actin staining is predominantly cortical (G) and FN is arranged in a fibrillar network (H). (I-L) Embryos expressing N-cad at stage 10 are similar to control embryos at stage 11.5; cells are polygonal (J), actin is organized at the cell cortex (K), and FN fibrils are assembled at the cell surface (L). Scale bar=25 μm in (L). (M,N). Saggital confocal sections of stage 10 embryos injected in one blastomere at the two cell stage with transcripts encoding a myc-epitope tagged N-cad (M), compared to an uninjected sibling control (N). Embryos were immunostained with antisera to FN (green) and mAb 9E10 to detect N-Cad-myc (red). (M, arrows) FN assembles in ectopic locations at the borders between non-expressing cells and cells expressing N-Cad. (N, arrows) In normal embryos FN is restricted to the surface facing the blastocoel cavity. Scale bar =50 μm in (N). (O, P). BCRs dissected from stage 11 embryos injected in one blastomere at the two cell stage with mRNA encoding (O) a dominant negative cadherin construct consisting of the IL2 receptor extracellular domain fused to the C-cad transmembrane and cytoplasmic domains or, (P) uninjected sibling control embryos. Isolated BCRs were immunostained with pAb32 to FN (green) and a mAb to the IL2 receptor (red). (O) FN matrix is absent from cells (red) expressing the dominant negative cadherin while, (O, P) FN fibril assembly is underway in control cells at this stage. Scale bar =50μm in (P). (Q) Western blot of cell surface levels of C-cad and β1 integrins in intact BCRs over expressing C-cad or dn C-cad.
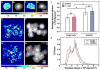
Figure 3. Traction force mapping of single vs. clustered cells on FN
(A-H) BCR cells on FN. (A-B, E-F) control cells and (C-D, G-H) cells overexpressing C-Cad. (A,C,E,G) Traction stress maps and (B,D,F,H) phase contrast images of same field. Colors express the magnitude of traction stress (× 10 4 dyn/cm2) mapped to cell regions. Note that the range of stresses represented by the color scale in (A-D) is different from scale in (E-H). Scale bars = 25 μm. (I) Cell-cell contact increases the average traction stress on FN exerted by both control cells and cells over expressing C-cad. Average traction stress per cell (+/-S.D.). (*) denotes statistical significance (p < 0.001). (ns) not significant. (J) Distribution of traction stress comparing single and clustered cells over expressing C-cad on FN substrates. Vertical axis represents percentage of total pixels with a certain stress value. Data derived from a total of 30 individual cell measurements (groups of 4-9 cells/cluster) per condition. Single cell data in (I) derived from 9 cells (3 separate experiments).
Figure 4. Rac activity is required for cadherin to promote FN assembly
Embryos injected with mRNA encoding N-cadherin and either (A-C) dominant negative RacN17 or (D-F) RhoN19 were fixed at stage 10. BCRs immunostained with (A,D,C,F, green) anti-FN pAb 32 or, (B,E,C,F, red) mAb 9E10 to detect areas of myc epitope-tagged N-cad. Co-expression of dominant negative Rac but not dominant negative Rho inhibited the fibril promoting effects of N-cad expression (compare A-C to D-F). Bar=25μm. (G) Western blot analysis of expression levels of myc epitope-tagged RacN17 Rac and RhoN19. β-actin used as loading control.
Figure 5. Rac and Pak activity are required for FN fibril assembly
(A-D) Confocal images of BCRs from stage 11.5 embryos expressing dominant negative Rac N17 and immunolabeled with (A green, B) pAb32 to FN, (A blue, D) mAb 9E10 to detect cells expressing myc epitope tagged RacN17 and, (A red, C) phalloidin. Cells expressing RacN17 are unable to assemble FN fibrils. Actin filaments are detected throughout the cytoplasm and are no longer enriched at the periphery of cells. (E-H) Control or (I-L) inhibitory Pak peptides were delivered at blastula stage 8 by intrablastocoelic injection and the embryos cultured to late gastrula stage. (E-G, I-K) Confocal images of stage 11.5 BCRs immunostained as in (A-C). Inhibition of both FN fibril assembly and dramatic reorganization of the actin cytoskeleton was observed in embryos injected with the Pak inhibitory peptide (compare F,G to J,K). (H) Phospho-MLC (pMLC) was detected at cell peripheries in stage-matched sibling embryos injected with control peptide. (L) pMLC was greatly reduced in Pak peptide injected BCR cells. (M) Western blot of embryo lysates from control (-) and Pak peptide (+) injected embryos. β-actin included as loading control. (N) Embryos injected with control peptide develop normally while (O) embryos injected with the PAK inhibitory peptide were shortened along the anterior posterior axis. (P-S) Confocal images of BCRs from stage 11.5 embryos immunostained for (P-green, Q) FN, and (P-red, S) phospho-Pak and, (R) F-actin with phalloidin. (P) Phosphorylated Pak detected as punctae that extensively colocalize with FN fibrils. Scale bar in (A) and (P) =25μm. Scale bar in (N) =1 mm.
Figure 6. Tissue tension regulates FN assembly
(A) Embryos were punctured with a fine glass pipette open at both ends prior to the onset of gastrulation (stage 10), and left in place until stage 11.5 with exogenous FN present in the culture medium. (B) Sibling control embryos were cultured under the same conditions without puncturing. BCRs were fixed and immunostained (C,D) using mAb 4H2, and (E,F) pAb 881 to detect FN and α5 integrin, respectively. (C) FN is detected at cell edges in punctured embryos but (D) arranged in fibrils in controls. (E) α5 integrins localized to cell membranes in punctured embryos as in controls (F), but control cells are arranged in a more polygonal pattern. Scale bar in (F) =50 μm. (G-J) Increased tension on the BCR promotes assembly. Individual mid-blastula (st. 8) embryos were (G) forced into a nitex grid or (H) placed on top of the grid and cultured until stage 10 and fixed. (I) FN fibrils are precociously assembled on the BCR in a pattern that corresponds to the perimeter of the nitex grid where the embryo is compressed. (J) pre-fibrillar FN is localized to cell edges in controls, typical of normal embryos at this stage. Scale bar in (J) =75 μm.
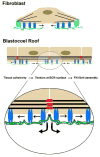
Figure 7. Model for regulation of FN assembly in tissues
The current view of FN matrix assembly is based on observations of cells (e.g., fibroblasts) in culture. The cell is anchored to the actin cytoskeleton via integrins in focal adhesions (light blue). An increase in local mechanical tension is transmitted to α5β1 integrins (blue) with bound dimeric FNs (green) and as integrin/FN complexes move centripetally (arrows) from focal adhesions, cryptic sites in FN are exposed that promote FN-FN assembly. On the BCR and in epithelia, the analogous role of the focal adhesion in culture is supplanted by associations of the actin cytoskeleton with cadherins (red) at adherens junctions. Local mechanical tension is transmitted to α5β1 with bound FN dimers. Cryptic sites in FN are exposed and promote FN-FN assembly as integrin/FN complexes move centripetally (arrows) from sites of cell-cell contact and across the free surfaces of adjacent cells in a Rac/Pak dependent process. It is possible that a single FN dimer could be bound to integrins on adjacent cells moving in opposite directions or to two integrins within a single cell, the latter of which is more similar to movement out of the focal contact in cultured cells.
Comment in
- Tightening the connections between cadherins and fibronectin matrix.
Hunt GC, Schwarzbauer JE. Hunt GC, et al. Dev Cell. 2009 Mar;16(3):327-8. doi: 10.1016/j.devcel.2009.02.013. Dev Cell. 2009. PMID: 19289079
Similar articles
- Tightening the connections between cadherins and fibronectin matrix.
Hunt GC, Schwarzbauer JE. Hunt GC, et al. Dev Cell. 2009 Mar;16(3):327-8. doi: 10.1016/j.devcel.2009.02.013. Dev Cell. 2009. PMID: 19289079 - N-cadherin cell-cell adhesion complexes are regulated by fibronectin matrix assembly.
Lefort CT, Wojciechowski K, Hocking DC. Lefort CT, et al. J Biol Chem. 2011 Jan 28;286(4):3149-60. doi: 10.1074/jbc.M110.115733. Epub 2010 Nov 17. J Biol Chem. 2011. PMID: 21084302 Free PMC article. - Multicellular computer simulation of morphogenesis: blastocoel roof thinning and matrix assembly in Xenopus laevis.
Longo D, Peirce SM, Skalak TC, Davidson L, Marsden M, Dzamba B, DeSimone DW. Longo D, et al. Dev Biol. 2004 Jul 1;271(1):210-22. doi: 10.1016/j.ydbio.2004.03.021. Dev Biol. 2004. PMID: 15196962 - Modulation of cell-extracellular matrix interactions.
Sechler JL, Corbett SA, Wenk MB, Schwarzbauer JE. Sechler JL, et al. Ann N Y Acad Sci. 1998 Oct 23;857:143-54. doi: 10.1111/j.1749-6632.1998.tb10114.x. Ann N Y Acad Sci. 1998. PMID: 9917839 Review. - The ins and outs of fibronectin matrix assembly.
Wierzbicka-Patynowski I, Schwarzbauer JE. Wierzbicka-Patynowski I, et al. J Cell Sci. 2003 Aug 15;116(Pt 16):3269-76. doi: 10.1242/jcs.00670. J Cell Sci. 2003. PMID: 12857786 Review.
Cited by
- Vinculin is required for cell polarization, migration, and extracellular matrix remodeling in 3D collagen.
Thievessen I, Fakhri N, Steinwachs J, Kraus V, McIsaac RS, Gao L, Chen BC, Baird MA, Davidson MW, Betzig E, Oldenbourg R, Waterman CM, Fabry B. Thievessen I, et al. FASEB J. 2015 Nov;29(11):4555-67. doi: 10.1096/fj.14-268235. Epub 2015 Jul 20. FASEB J. 2015. PMID: 26195589 Free PMC article. - VANGL2 regulates membrane trafficking of MMP14 to control cell polarity and migration.
Williams BB, Cantrell VA, Mundell NA, Bennett AC, Quick RE, Jessen JR. Williams BB, et al. J Cell Sci. 2012 May 1;125(Pt 9):2141-7. doi: 10.1242/jcs.097964. Epub 2012 Feb 22. J Cell Sci. 2012. PMID: 22357946 Free PMC article. - O-fucosylation of thrombospondin type 1 repeats restricts epithelial to mesenchymal transition (EMT) and maintains epiblast pluripotency during mouse gastrulation.
Du J, Takeuchi H, Leonhard-Melief C, Shroyer KR, Dlugosz M, Haltiwanger RS, Holdener BC. Du J, et al. Dev Biol. 2010 Oct 1;346(1):25-38. doi: 10.1016/j.ydbio.2010.07.008. Epub 2010 Jul 14. Dev Biol. 2010. PMID: 20637190 Free PMC article. - Osmotic Gradients in Epithelial Acini Increase Mechanical Tension across E-cadherin, Drive Morphogenesis, and Maintain Homeostasis.
Narayanan V, Schappell LE, Mayer CR, Duke AA, Armiger TJ, Arsenovic PT, Mohan A, Dahl KN, Gleghorn JP, Conway DE. Narayanan V, et al. Curr Biol. 2020 Feb 24;30(4):624-633.e4. doi: 10.1016/j.cub.2019.12.025. Epub 2020 Jan 23. Curr Biol. 2020. PMID: 31983640 Free PMC article. - Wnt signaling related transcripts and their relationship to energy metabolism in C2C12 myoblasts under temperature stress.
Risha MA, Ali A, Siengdee P, Trakooljul N, Haack F, Dannenberger D, Wimmers K, Ponsuksili S. Risha MA, et al. PeerJ. 2021 Jun 14;9:e11625. doi: 10.7717/peerj.11625. eCollection 2021. PeerJ. 2021. PMID: 34178477 Free PMC article.
References
- Arthur WT, Noren NK, Burridge K. Regulation of Rho family GTPases by cell-cell and cell-matrix adhesion. Biol Res. 2002;35:239–246. - PubMed
- Bershadsky A. Magic Touch: how does cell-cell adhesion trigger actin assembly? Trends in Cell Biology. 2004;14:589–593. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous