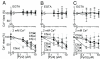Selective and direct inhibition of TRPC3 channels underlies biological activities of a pyrazole compound - PubMed (original) (raw)
. 2009 Mar 31;106(13):5400-5.
doi: 10.1073/pnas.0808793106. Epub 2009 Mar 16.
Kenta Kato, Motohiro Nishida, Kazuhiro Mio, Takuro Numaga, Yuichi Sawaguchi, Takashi Yoshida, Minoru Wakamori, Emiko Mori, Tomohiro Numata, Masakazu Ishii, Hiroki Takemoto, Akio Ojida, Kenta Watanabe, Aya Uemura, Hitoshi Kurose, Takashi Morii, Tsutomu Kobayashi, Yoji Sato, Chikara Sato, Itaru Hamachi, Yasuo Mori
Affiliations
- PMID: 19289841
- PMCID: PMC2664023
- DOI: 10.1073/pnas.0808793106
Selective and direct inhibition of TRPC3 channels underlies biological activities of a pyrazole compound
Shigeki Kiyonaka et al. Proc Natl Acad Sci U S A. 2009.
Abstract
Canonical transient receptor potential (TRPC) channels control influxes of Ca(2+) and other cations that induce diverse cellular processes upon stimulation of plasma membrane receptors coupled to phospholipase C (PLC). Invention of subtype-specific inhibitors for TRPCs is crucial for distinction of respective TRPC channels that play particular physiological roles in native systems. Here, we identify a pyrazole compound (Pyr3), which selectively inhibits TRPC3 channels. Structure-function relationship studies of pyrazole compounds showed that the trichloroacrylic amide group is important for the TRPC3 selectivity of Pyr3. Electrophysiological and photoaffinity labeling experiments reveal a direct action of Pyr3 on the TRPC3 protein. In DT40 B lymphocytes, Pyr3 potently eliminated the Ca(2+) influx-dependent PLC translocation to the plasma membrane and late oscillatory phase of B cell receptor-induced Ca(2+) response. Moreover, Pyr3 attenuated activation of nuclear factor of activated T cells, a Ca(2+)-dependent transcription factor, and hypertrophic growth in rat neonatal cardiomyocytes, and in vivo pressure overload-induced cardiac hypertrophy in mice. These findings on important roles of native TRPC3 channels are strikingly consistent with previous genetic studies. Thus, the TRPC3-selective inhibitor Pyr3 is a powerful tool to study in vivo function of TRPC3, suggesting a pharmaceutical potential of Pyr3 in treatments of TRPC3-related diseases such as cardiac hypertrophy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Selective inhibition of TRPC3-mediated Ca2+ influx by Pyr3. (A) Chemical structure of Pyr3. (B–E) Concentration-dependent inhibitory action of Pyr3 on ATP receptor-induced (B and C) or mAChR-induced (D and E) induced Ca2+ influx via TRPCs. (B and D) Average time courses of Ca2+ responses induced by 100 μM ATP in HEK293 cells (B) or by 100 μM CCh in HEK293T cells (D) transfected with TRPCs at indicated Pyr3 concentrations. (C and E) Percentage peak [Ca2+]i rises in Ca2+-free, 0.5 mM EGTA-containing (Upper) or 2 mM Ca2+-containing (Lower) external solution compared with control responses without Pyr3 (n = 33–104). (F) Pyr3 Inhibition of Ca2+ influx via OAG-activated TRPC3. Average time courses of Ca2+ responses induced by 10 μM OAG at indicated Pyr3 concentrations in TRPC3-transfected HEK293 cells (Left). Percentage peak [Ca2+]i rises in 2 mM Ca2+ solution (Right) (n = 19–37).
Fig. 2.
Inhibition of TRPCs by Pyr2, Pyr4, or Pyr5. (A–C) Concentration-dependent inhibitory action of Pyr2 (A), Pyr4 (B), or Pyr5 (C) on Ca2+ influx induced by 100 μM ATP via TRPCs. Percentage peak [Ca2+]i rises in Ca2+-free, 0.5 mM EGTA- (Upper) or 2 mM Ca2+-containing (Lower) external solution compared with control responses without drugs (n = 18–66).
Fig. 3.
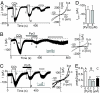
mAChR-activated TRPC3 current is suppressed by extracellular application of Pyr3. (A–C) Traces of ionic currents induced by 60 μM CCh at a holding potential of −50 mV in TRPC3-transfected HEK293 cells (Left). I–V relationships obtained by subtracting the currents evoked by the voltage-ramps before activation of channels (a and c) from those after activation (current traces b and d) (Right). (B) Three μM Pyr3 is added 1.5 min before second stimulation of CCh into the external solution. (C) Three μM Pyr3 is added in internal solution before the recordings, and then external Pyr3 is also applied 1.5 min before second CCh stimulation. (D) Average current amplitudes of the first response at −50 mV in the presence (n = 7) or absence (n = 8) of 3 μM Pyr3 in the internal solution. (E) Concentration-dependent inhibitory action of Pyr3, using the testing paradigm depicted in B and C. The amplitude of the second response was normalized to that of the first (peak2/peak1) (n = 4–8). **, P < 0.01 and ***, P < 0.001 vs. 0 μM Pyr3.
Fig. 4.
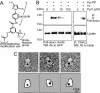
Photochemical cross-linking of TRPC3 with Pyr-PP. (A) Chemical structure of Pyr-PP. (B) Pyr-PP directly binds TRPC3. After P-PALM, TRPC3-GFP proteins are detected with anti-GFP antibody by Western blot analysis (WB) in avidin pull-down samples. The photochemical Pyr-PP cross-linking of TRPC3 is inhibited by 3-min preincubation and subsequent coincubation with Pyr3 (10 or 100 μM) (Left). After P-PALM, the incorporation of the Pyr-PP-ARP adduct is detected with anti-biotin antibody by WB in immunoprecipitated (IP) samples with anti-TRPC3 antibody (Right). (C) Electron microscopic visualization of negatively stained TRPC3 after P-PALM with gold nanoparticles. Streptavidin-gold conjugate is attached to labeled-TRPC3 via biotin-labeling site.
Fig. 5.
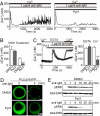
Pyr3 inhibits native TRPC3 channels and downstream responses in DT40 B lymphocytes. (A and B) Inhibitory action of 0.3 μM Pyr3 on Ca2+ oscillation upon BCR stimulation with 1 μg/mL anti-IgM. (A) Representative time courses with (Right) or without of Pyr3 (Left). (B) Peak [Ca2+]i rises at initial and sustained phases (30–40 min). (C) Inhibitory action of 1 μM Pyr3 on BCR-induced Ca2+ influx. Average time courses (Left). Peak [Ca2+]i rises in Ca2+-free, 0.5 mM EGTA- or 2 mM Ca2+-containing solution (n = 42–49) (Right). (D) Confocal fluorescence images indicating PM translocation of PLCγ2-EYFP upon BCR-stimulation with 10 μg/mL anti-IgM. Three μM Pyr3 is applied 10 min before BCR stimulation. (E) Effects of Pyr3 on ERK activation induced by BCR stimulation (5 μg/mL anti-IgM). Three μM Pyr3 is applied 10 min before BCR stimulation. Cells are analyzed by WB, using anti-phospho-ERK2 antibody. ***, P < 0.001 vs. DMSO.
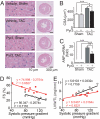
Fig. 6.
Potent suppressive effects of Pyr3 on cardiac hypertrophy induced by pressure overload in mice. (A–C) Effects of Pyr3 on 1-week TAC-induced concentric hypertrophy. (A) H&E-stained mid transverse sections of hearts isolated from sham- and TAC-operated mice. (B and C) Effects of Pyr3 on the increase in cross-sectional areas (CSA) (B) and ANP mRNA expressions (C). *, P < 0.05, **, P < 0.01, and ***, P < 0.001. (D and E) Effects of Pyr3 on 6-weeks TAC-induced dilated hypertrophy. Scattergram of systolic pressure gradient vs. FS, a surrogate of systolic function (D) and LVW/TL (E) in TAC-operated mice with (red) and without (black) treatment with Pyr3. Pyr3 significantly shifts relationships upward in FS (P < 0.001) and downward in LVW/TL (P < 0.01).
Similar articles
- [Pharmacological properties of novel TRPC channel inhibitors].
Kiyonaka S, Kato K, Nishida M, Mori Y. Kiyonaka S, et al. Yakugaku Zasshi. 2010 Mar;130(3):303-11. doi: 10.1248/yakushi.130.303. Yakugaku Zasshi. 2010. PMID: 20190514 Review. Japanese. - Isoproterenol-induced hypertrophy of neonatal cardiac myocytes and H9c2 cell is dependent on TRPC3-regulated CaV1.2 expression.
Han JW, Kang C, Kim Y, Lee MG, Kim JY. Han JW, et al. Cell Calcium. 2020 Dec;92:102305. doi: 10.1016/j.ceca.2020.102305. Epub 2020 Oct 6. Cell Calcium. 2020. PMID: 33069962 - A TRPC3 blocker, ethyl-1-(4-(2,3,3-trichloroacrylamide)phenyl)-5-(trifluoromethyl)-1H-pyrazole-4-carboxylate (Pyr3), prevents stent-induced arterial remodeling.
Koenig S, Schernthaner M, Maechler H, Kappe CO, Glasnov TN, Hoefler G, Braune M, Wittchow E, Groschner K. Koenig S, et al. J Pharmacol Exp Ther. 2013 Jan;344(1):33-40. doi: 10.1124/jpet.112.196832. Epub 2012 Sep 25. J Pharmacol Exp Ther. 2013. PMID: 23010361 - Regulation of canonical transient receptor potential (TRPC) channel function by diacylglycerol and protein kinase C.
Venkatachalam K, Zheng F, Gill DL. Venkatachalam K, et al. J Biol Chem. 2003 Aug 1;278(31):29031-40. doi: 10.1074/jbc.M302751200. Epub 2003 Apr 29. J Biol Chem. 2003. PMID: 12721302 - Pharmacological modulation of diacylglycerol-sensitive TRPC3/6/7 channels.
Harteneck C, Gollasch M. Harteneck C, et al. Curr Pharm Biotechnol. 2011 Jan 1;12(1):35-41. doi: 10.2174/138920111793937943. Curr Pharm Biotechnol. 2011. PMID: 20932261 Free PMC article. Review.
Cited by
- New Frontiers on ER Stress Modulation: Are TRP Channels the Leading Actors?
Vestuto V, Di Sarno V, Musella S, Di Dona G, Moltedo O, Gomez-Monterrey IM, Bertamino A, Ostacolo C, Campiglia P, Ciaglia T. Vestuto V, et al. Int J Mol Sci. 2022 Dec 22;24(1):185. doi: 10.3390/ijms24010185. Int J Mol Sci. 2022. PMID: 36613628 Free PMC article. Review. - Transient Receptor Potential Canonical 3 and Nuclear Factor of Activated T Cells C3 Signaling Pathway Critically Regulates Myocardial Fibrosis.
Saliba Y, Jebara V, Hajal J, Maroun R, Chacar S, Smayra V, Abramowitz J, Birnbaumer L, Farès N. Saliba Y, et al. Antioxid Redox Signal. 2019 Jun 1;30(16):1851-1879. doi: 10.1089/ars.2018.7545. Epub 2018 Nov 29. Antioxid Redox Signal. 2019. PMID: 30318928 Free PMC article. - Progress on role of ion channels of cardiac fibroblasts in fibrosis.
Xing C, Bao L, Li W, Fan H. Xing C, et al. Front Physiol. 2023 Mar 9;14:1138306. doi: 10.3389/fphys.2023.1138306. eCollection 2023. Front Physiol. 2023. PMID: 36969589 Free PMC article. Review. - SLO2.1/NALCN a sodium signaling complex that regulates uterine activity.
Ferreira JJ, Amazu C, Puga-Molina LC, Ma X, England SK, Santi CM. Ferreira JJ, et al. iScience. 2021 Oct 2;24(11):103210. doi: 10.1016/j.isci.2021.103210. eCollection 2021 Nov 19. iScience. 2021. PMID: 34746693 Free PMC article. - Role of TRPC1 and TRPC3 channels in contraction and relaxation of mouse thoracic aorta.
Kochukov MY, Balasubramanian A, Noel RC, Marrelli SP. Kochukov MY, et al. J Vasc Res. 2013;50(1):11-20. doi: 10.1159/000342461. Epub 2012 Oct 23. J Vasc Res. 2013. PMID: 23095462 Free PMC article.
References
- Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential channels in disease. Physiol Rev. 2007;87:165–217. - PubMed
- Hardie RC, Minke B. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron. 1992;8:643–651. - PubMed
- Zhu X, et al. trp, a novel mammalian gene family essential for agonist-activated capacitative Ca2+ entry. Cell. 1996;85:661–671. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous