Alcohol-induced protein hyperacetylation: mechanisms and consequences - PubMed (original) (raw)
Review
Alcohol-induced protein hyperacetylation: mechanisms and consequences
Blythe D Shepard et al. World J Gastroenterol. 2009.
Abstract
Although the clinical manifestations of alcoholic liver disease are well-described, little is known about the molecular basis of liver injury. Recent studies have indicated that ethanol exposure induces global protein hyperacetylation. This reversible, post-translational modification on the epsilon-amino groups of lysine residues has been shown to modulate multiple, diverse cellular processes ranging from transcriptional activation to microtubule stability. Thus, alcohol-induced protein hyperacetylation likely leads to major physiological consequences that contribute to alcohol-induced hepatotoxicity. Lysine acetylation is controlled by the activities of two opposing enzymes, histone acetyltransferases and histone deacetylases. Currently, efforts are aimed at determining which enzymes are responsible for the increased acetylation of specific substrates. However, the greater challenge will be to determine the physiological ramifications of protein hyperacetylation and how they might contribute to the progression of liver disease. In this review, we will first list and discuss the proteins known to be hyperacetylated in the presence of ethanol. We will then describe what is known about the mechanisms leading to increased protein acetylation and how hyperacetylation may perturb hepatic function.
Figures
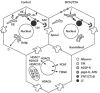
Figure 1
Alcohol-induced defects in protein trafficking can be explained by increased microtubule acetylation and stability. Cargo internalized from the basolateral plasma membrane is delivered to the early endosome (EE) and sorted into at least four different pathways. They are recycled directly back to the plasma membrane (e.g. ASGP-R), delivered to the recycling endosome (RE) before recycling back to the plasma membrane (e.g. Tf-R), delivered to lysosomes (lys) (e.g. LY) or to the apical plasma membrane via the sub-apical compartment (SAC) (e.g. APN, pIgA-R and 5’NT). Albumin secretion is also indicated. In ethanol (EtOH) or TSA treated cells, albumin secretion and the internalization of APN, pIgA-R, Tf-R and ASGP-R is impaired whereas the fluid-phase delivery of LY to lysosomes or the raft/caveolae-mediated internalization of 5’NT and CTxB are not changed. In the lower hepatocyte, the various deacetylases and acetyltransferases that may play a role in the acetylation of nonnuclear proteins are indicated.
Similar articles
- Ethanol metabolism by alcohol dehydrogenase or cytochrome P450 2E1 differentially impairs hepatic protein trafficking and growth hormone signaling.
Doody EE, Groebner JL, Walker JR, Frizol BM, Tuma DJ, Fernandez DJ, Tuma PL. Doody EE, et al. Am J Physiol Gastrointest Liver Physiol. 2017 Dec 1;313(6):G558-G569. doi: 10.1152/ajpgi.00027.2017. Epub 2017 Sep 1. Am J Physiol Gastrointest Liver Physiol. 2017. PMID: 28864499 Free PMC article. - In Vivo Acute on Chronic Ethanol Effects in Liver: A Mouse Model Exhibiting Exacerbated Injury, Altered Metabolic and Epigenetic Responses.
Shukla SD, Aroor AR, Restrepo R, Kharbanda KK, Ibdah JA. Shukla SD, et al. Biomolecules. 2015 Nov 20;5(4):3280-94. doi: 10.3390/biom5043280. Biomolecules. 2015. PMID: 26610587 Free PMC article. - Chronic ethanol consumption induces global hepatic protein hyperacetylation.
Shepard BD, Tuma DJ, Tuma PL. Shepard BD, et al. Alcohol Clin Exp Res. 2010 Feb;34(2):280-91. doi: 10.1111/j.1530-0277.2009.01091.x. Epub 2009 Nov 24. Alcohol Clin Exp Res. 2010. PMID: 19951295 Free PMC article. - HATs and HDACs: from structure, function and regulation to novel strategies for therapy and prevention.
Yang XJ, Seto E. Yang XJ, et al. Oncogene. 2007 Aug 13;26(37):5310-8. doi: 10.1038/sj.onc.1210599. Oncogene. 2007. PMID: 17694074 Review. - Oxidative stress and alcoholic liver disease.
Wu D, Cederbaum AI. Wu D, et al. Semin Liver Dis. 2009 May;29(2):141-54. doi: 10.1055/s-0029-1214370. Epub 2009 Apr 22. Semin Liver Dis. 2009. PMID: 19387914 Review.
Cited by
- Age-related alterations in histone deacetylase expression in Purkinje neurons of ethanol-fed rats.
Khurana A, Dlugos CA. Khurana A, et al. Brain Res. 2017 Nov 15;1675:8-19. doi: 10.1016/j.brainres.2017.08.026. Epub 2017 Aug 30. Brain Res. 2017. PMID: 28855102 Free PMC article. - Mitochondrial acetylome analysis in a mouse model of alcohol-induced liver injury utilizing SIRT3 knockout mice.
Fritz KS, Galligan JJ, Hirschey MD, Verdin E, Petersen DR. Fritz KS, et al. J Proteome Res. 2012 Mar 2;11(3):1633-43. doi: 10.1021/pr2008384. Epub 2012 Feb 21. J Proteome Res. 2012. PMID: 22309199 Free PMC article. - Hepatic microtubule acetylation and stability induced by chronic alcohol exposure impair nuclear translocation of STAT3 and STAT5B, but not Smad2/3.
Fernandez DJ, Tuma DJ, Tuma PL. Fernandez DJ, et al. Am J Physiol Gastrointest Liver Physiol. 2012 Dec 15;303(12):G1402-15. doi: 10.1152/ajpgi.00071.2012. Epub 2012 Oct 11. Am J Physiol Gastrointest Liver Physiol. 2012. PMID: 23064763 Free PMC article. - Ethanol sensitizes mitochondria to the permeability transition by inhibiting deacetylation of cyclophilin-D mediated by sirtuin-3.
Shulga N, Pastorino JG. Shulga N, et al. J Cell Sci. 2010 Dec 1;123(Pt 23):4117-27. doi: 10.1242/jcs.073502. Epub 2010 Nov 9. J Cell Sci. 2010. PMID: 21062897 Free PMC article. Retracted. - Acetyl-CoA carboxylase 1-dependent lipogenesis promotes autophagy downstream of AMPK.
Gross AS, Zimmermann A, Pendl T, Schroeder S, Schoenlechner H, Knittelfelder O, Lamplmayr L, Santiso A, Aufschnaiter A, Waltenstorfer D, Ortonobes Lara S, Stryeck S, Kast C, Ruckenstuhl C, Hofer SJ, Michelitsch B, Woelflingseder M, Müller R, Carmona-Gutierrez D, Madl T, Büttner S, Fröhlich KU, Shevchenko A, Eisenberg T. Gross AS, et al. J Biol Chem. 2019 Aug 9;294(32):12020-12039. doi: 10.1074/jbc.RA118.007020. Epub 2019 Jun 17. J Biol Chem. 2019. PMID: 31209110 Free PMC article.
References
- Brooks PJ. DNA damage, DNA repair, and alcohol toxicity--a review. Alcohol Clin Exp Res. 1997;21:1073–1082. - PubMed
- Kenney WC. Acetaldehyde adducts of phospholipids. Alcohol Clin Exp Res. 1982;6:412–416. - PubMed
- Kenney WC. Formation of Schiff base adduct between acetaldehyde and rat liver microsomal phosphatidylethanolamine. Alcohol Clin Exp Res. 1984;8:551–555. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources