P2X4-receptor-mediated synthesis and release of brain-derived neurotrophic factor in microglia is dependent on calcium and p38-mitogen-activated protein kinase activation - PubMed (original) (raw)
Comparative Study
P2X4-receptor-mediated synthesis and release of brain-derived neurotrophic factor in microglia is dependent on calcium and p38-mitogen-activated protein kinase activation
Tuan Trang et al. J Neurosci. 2009.
Abstract
Microglia in the dorsal horn of the spinal cord are increasingly recognized as being crucial in the pathogenesis of pain hypersensitivity after injury to a peripheral nerve. It is known that P2X4 purinoceptors (P2X4Rs) cause the release of brain-derived neurotrophic factor (BDNF) from microglia, which is necessary for maintaining pain hypersensitivity after nerve injury. However, there is a critical gap in understanding how activation of microglial P2X4Rs leads to the release of BDNF. Here, we show that stimulating P2X4Rs with ATP evokes a biphasic release of BDNF from microglia: an early phase occurs within 5 min, whereas a late phase peaks 60 min after ATP stimulation. Concomitant with the late phase of release is an increased level of BDNF within the microglia. Both phases of BDNF release and the accumulation within the microglia are dependent on extracellular Ca(2+). The late phase of BDNF release and accumulation, but not the early phase of release, are suppressed by inhibiting transcription and translation, indicating that activation of P2X4R causes an initial release of a pre-existing pool of BDNF followed by an increase in de novo synthesis of BDNF. The release of BDNF is abolished by inhibiting SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor)-mediated exocytosis. Furthermore, we find that the P2X4R-evoked release and synthesis of BDNF are dependent on activation of p38-mitogen-activated protein kinase (MAPK). Together, our findings provide a unifying mechanism for pain hypersensitivity after peripheral nerve injury through P2X4R-evoked increase in Ca(2+) and activation of p38-MAPK leading to the synthesis and exocytotic release of BDNF from microglia.
Figures
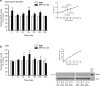
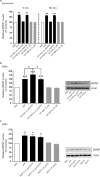
Figure 1.
ATP causes a biphasic release of BDNF and an increase in BDNF protein in rat primary microglial culture. a, Left, ELISA-based measurement of BDNF released into the supernatant at different time points after adding ATP. a, Right, A representative BDNF ELISA standard curve (_R_2 = 0.97); mean BDNF measurement for PBS and ATP (50 μ
m
) treated groups at 5 min and 60 min is indicated on the graph. b, Left, Western blot analysis of BDNF protein in microglial cell lysates after adding ATP. Band intensity was quantitated as mean gray value and normalized to PBS-treated control. b, Right, Representative standard curve derived from serial dilutions of primary microglial cell lysate proteins (_R_2 = 0.98). Representative Western blot of BDNF protein from microglial cell lysate. In each lane, 35 μg of protein was loaded. Each time point represents n = 6–8. Data are presented as mean percentage of PBS-treated control (±SEM). **p < 0.01 compared with PBS-treated control.
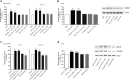
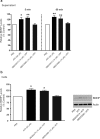
Figure 2.
Blocking or suppressing expression of microglial P2X4Rs attenuates the ATP-evoked release and the accumulation of BDNF. a, b, Microglia were pretreated with pharmacological antagonists of P2XRs: TNP–ATP (50 μ
m
) or PPADs (50 μ
m
). c, d, Microglia were transfected with P2X4R-targeting siRNA (600 ng) over 72 h; controls received vehicle-only or nontargeting siRNA (600 ng). a, c, Release of BDNF into the supernatant 5 min and 60 min after adding ATP (50 μ
m
) was measured by ELISA-based approach. b, d, BDNF protein expression 60 min after adding ATP was measured by Western blot analysis. The data are normalized to PBS control and expressed as percentage control (±SEM). In siRNA transfection experiments, the data are normalized to vehicle control. Each experimental group represents n = 4–7. *Represents significant difference from PBS or vehicle control; *p < 0.05; **p < 0.01.
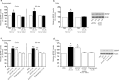
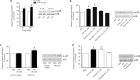
Figure 3.
ATP-evoked release and accumulation of BDNF is dependent on the presence of extracellular Ca2+. a, b, Microglia were stimulated with ATP (50 μ
m
) in extracellular solution containing Ca2+ (2 m
m
) or in extracellular solution with no added Ca2+. c, d, Microglia were treated with the Ca2+-ionophore A23187 (2 μ
m
) or with the sarcoplasmic reticulum/endoplasmic reticulum inhibitor thapsigargin (2 μ
m
). a, c, ELISA-based measurements of BDNF release at 5 min and 60 min after drug treatment. b, d, Western blot analysis of BDNF protein in microglial cell lysates 60 min after drug treatment. TNP–ATP was included in the extracellular solution in experiments using A23187 to prevent possible secondary activation of P2X4Rs by release of ATP from the microglia. Data are normalized to PBS control and presented as mean percentage of PBS control (±SEM). Each experimental group represents n = 5. *p < 0.05; **p < 0.01 compared with PBS-treated control.
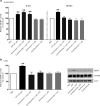
Figure 4.
Inhibiting transcription or translation prevents the ATP-evoked late phase of BDNF release and the concomitant increase in BDNF protein. Microglia were treated with the transcriptional inhibitor, actinomycin-D (1 μ
m
), or the translational inhibitor, cycloheximide (1 μ
m
). a, ELISA-based measurement of BDNF release 5 min and 60 min after ATP stimulation. b, Western blot analysis of BDNF protein in microglial cell lysates 60 min after ATP stimulation. Data are presented as mean percentage of PBS-treated control (±SEM). Each experimental group represents n = 5. **p < 0.01 compared with PBS-treated control.
Figure 5.
ATP-evoked release of BDNF from microglia is prevented by inhibiting SNARE-mediated exocytosis, and BDNF-TrkB signaling is not required for accumulation of BDNF within the microglia. Microglia were treated with TAT–NSF700 (5 μ
m
), an inhibitor of SNARE-mediated exocytosis, or control peptide TAT–NSF700scr (5 μ
m
) 60 min before ATP stimulation. a, ELISA-based measurement of BDNF release 5 min and 60 min after ATP stimulation. b, Western blot analysis of BDNF protein in microglial cell lysates 60 min after ATP stimulation. c, Western blot analysis of BDNF protein in microglial cells lysates treated with the BDNF-sequestering protein TrkB-Fc (5 μg/ml) or the control peptide IgG-Fc (5 μg/ml) at 60 min after ATP stimulation. Data normalized to PBS control and are presented as mean percentage of PBS control (±SEM). TAT–NSF experiments represent n = 6 for each experimental group. TrkB-Fc experiments represent n = 4 for each experimental group. *p < 0.05; **p < 0.01; ***p < 0.001 compared with PBS-treated control.
Figure 6.
Treatment with the p38-MAPK SB203580 suppresses ATP-evoked BDNF release and synthesis. Microglia were treated with the p38-MAPK inhibitor SB203580 (10 μ
m
) or its inactive analog SB202474 (10 μ
m
). a, ELISA-based measurement of BDNF release 5 min and 60 min after ATP (50 μ
m
) stimulation. b, Western blot analysis of BDNF protein in microglial cell lysates 60 min after ATP stimulation. Data are presented as mean percentage of PBS-treated control (±SEM). Each experimental group represents n = 5. *p < 0.05; **p < 0.01 compared with PBS-treated control.
Figure 7.
ATP-induced activation of p38-MAPK is prevented by TNP–ATP, SB203580, or in extracellular solution without Ca2+. a, Western blot analysis of phospho-p38-MAPK expression in microglial cell lysates 5 min and 60 min after ATP (50 μ
m
) stimulation. b, Effect of P2XR antagonists, PPADS (50 μ
m
) or TNP–ATP (50 μ
m
), and p38-MAPK inhibitor SB203580 (10 μ
m
), on phospho-p38-MAPK levels 60 min after ATP stimulation. c, Phospho-p38-MAPK expression in extracellular solution containing Ca2+ (2 m
m
) or in extracellular solution with no added Ca2+ 60 min after ATP stimulation. d, Effect of treatment with the Ca2+-ionophore A23187 (2 μ
m
) or the sarcoplasmic reticulum/endoplasmic reticulum inhibitor thapsigargin (2 μ
m
) on phospho-p38-MAPK expression at 60 min after treatment. The data are normalized to PBS control and expressed as percentage control (±SEM). Each experimental group represents n = 5. *p < 0.01 compared with PBS-treated control.
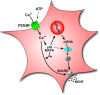
Figure 8.
Model illustrating ATP-evoked BDNF release and synthesis from microglia. Stimulation of P2X4Rs by ATP causes the SNARE-mediated release and synthesis of BDNF that is dependent on extracellular Ca2+ and activation of p38-MAPK. The early phase of release is from a pool of pre-existing BDNF, whereas the late phase of BDNF release and the accumulation of BDNF in the microglia are dependent on transcription and translation of de novo BDNF.
Similar articles
- Microglia in Neuropathic Pain.
Inoue K. Inoue K. Adv Neurobiol. 2024;37:399-403. doi: 10.1007/978-3-031-55529-9_22. Adv Neurobiol. 2024. PMID: 39207704 Review. - Microglia P2X4R-BDNF signalling contributes to central sensitization in a recurrent nitroglycerin-induced chronic migraine model.
Long T, He W, Pan Q, Zhang S, Zhang D, Qin G, Chen L, Zhou J. Long T, et al. J Headache Pain. 2020 Jan 14;21(1):4. doi: 10.1186/s10194-019-1070-4. J Headache Pain. 2020. PMID: 31937253 Free PMC article. - Toll-like receptor-4/p38 MAPK signaling in the dorsal horn contributes to P2X4 receptor activation and BDNF over-secretion in cancer induced bone pain.
Meng XW, Gao JL, Zuo JL, Wang LN, Liu SL, Jin XH, Yao M, Namaka M. Meng XW, et al. Neurosci Res. 2017 Dec;125:37-45. doi: 10.1016/j.neures.2017.06.006. Epub 2017 Jun 28. Neurosci Res. 2017. PMID: 28668500 - [The mechanism and control of neuropathic pain].
Inoue K. Inoue K. Rinsho Shinkeigaku. 2009 Nov;49(11):779-82. doi: 10.5692/clinicalneurol.49.779. Rinsho Shinkeigaku. 2009. PMID: 20030208 Review. Japanese.
Cited by
- BK channels in microglia are required for morphine-induced hyperalgesia.
Hayashi Y, Morinaga S, Zhang J, Satoh Y, Meredith AL, Nakata T, Wu Z, Kohsaka S, Inoue K, Nakanishi H. Hayashi Y, et al. Nat Commun. 2016 May 31;7:11697. doi: 10.1038/ncomms11697. Nat Commun. 2016. PMID: 27241733 Free PMC article. - Microglia during development and aging.
Harry GJ. Harry GJ. Pharmacol Ther. 2013 Sep;139(3):313-26. doi: 10.1016/j.pharmthera.2013.04.013. Epub 2013 Apr 30. Pharmacol Ther. 2013. PMID: 23644076 Free PMC article. Review. - Microglia and Inhibitory Circuitry in the Medullary Dorsal Horn: Laminar and Time-Dependent Changes in a Trigeminal Model of Neuropathic Pain.
García-Magro N, Martin YB, Negredo P, Zafra F, Avendaño C. García-Magro N, et al. Int J Mol Sci. 2021 Apr 27;22(9):4564. doi: 10.3390/ijms22094564. Int J Mol Sci. 2021. PMID: 33925417 Free PMC article. - Microglia in Neuropathic Pain.
Inoue K. Inoue K. Adv Neurobiol. 2024;37:399-403. doi: 10.1007/978-3-031-55529-9_22. Adv Neurobiol. 2024. PMID: 39207704 Review. - Microglial BDNF, PI3K, and p-ERK in the Spinal Cord Are Suppressed by Pulsed Radiofrequency on Dorsal Root Ganglion to Ease SNI-Induced Neuropathic Pain in Rats.
Xu X, Fu S, Shi X, Liu R. Xu X, et al. Pain Res Manag. 2019 Apr 28;2019:5948686. doi: 10.1155/2019/5948686. eCollection 2019. Pain Res Manag. 2019. PMID: 31182984 Free PMC article.
References
- Altar CA, DiStefano PS. Neurotrophin trafficking by anterograde transport. Trends Neurosci. 1998;21:433–437. - PubMed
- Bader MF, Taupenot L, Ulrich G, Aunis D, Ciesielski-Treska J. Bacterial endotoxin induces [Ca2+]i transients and changes the organization of actin in microglia. Glia. 1994;11:336–344. - PubMed
- Blanquet PR. Identification of two persistently activated neurotrophin-regulated pathways in rat hippocampus. Neuroscience. 2000;95:705–719. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous