Adaptive designs for randomized trials in public health - PubMed (original) (raw)
Review
Adaptive designs for randomized trials in public health
C Hendricks Brown et al. Annu Rev Public Health. 2009.
Abstract
In this article, we present a discussion of two general ways in which the traditional randomized trial can be modified or adapted in response to the data being collected. We use the term adaptive design to refer to a trial in which characteristics of the study itself, such as the proportion assigned to active intervention versus control, change during the trial in response to data being collected. The term adaptive sequence of trials refers to a decision-making process that fundamentally informs the conceptualization and conduct of each new trial with the results of previous trials. Our discussion below investigates the utility of these two types of adaptations for public health evaluations. Examples are provided to illustrate how adaptation can be used in practice. From these case studies, we discuss whether such evaluations can or should be analyzed as if they were formal randomized trials, and we discuss practical as well as ethical issues arising in the conduct of these new-generation trials.
Figures
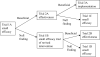
Figure 1
Adaptive designs leading to early stopping or modifying allocation probabilities.
Figure 2
A typical sequencing of trials.
Figure 3
Cumulative adaptive trials with continuous quality improvement.
Similar articles
- The future of Cochrane Neonatal.
Soll RF, Ovelman C, McGuire W. Soll RF, et al. Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834 - Overview, hurdles, and future work in adaptive designs: perspectives from a National Institutes of Health-funded workshop.
Coffey CS, Levin B, Clark C, Timmerman C, Wittes J, Gilbert P, Harris S. Coffey CS, et al. Clin Trials. 2012 Dec;9(6):671-80. doi: 10.1177/1740774512461859. Clin Trials. 2012. PMID: 23250942 Free PMC article. - Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.
Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, Moraleda C, Rogers L, Daniels K, Green P. Crider K, et al. Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article. - Essential Ingredients and Innovations in the Design and Analysis of Group-Randomized Trials.
Murray DM, Taljaard M, Turner EL, George SM. Murray DM, et al. Annu Rev Public Health. 2020 Apr 2;41:1-19. doi: 10.1146/annurev-publhealth-040119-094027. Epub 2019 Dec 23. Annu Rev Public Health. 2020. PMID: 31869281 Review. - Overview of the epidemiology methods and applications: strengths and limitations of observational study designs.
Colditz GA. Colditz GA. Crit Rev Food Sci Nutr. 2010;50 Suppl 1(s1):10-2. doi: 10.1080/10408398.2010.526838. Crit Rev Food Sci Nutr. 2010. PMID: 21132580 Free PMC article.
Cited by
- Adaptive designs in critical care trials: a simulation study.
Li W, Cornelius V, Finfer S, Venkatesh B, Billot L. Li W, et al. BMC Med Res Methodol. 2023 Oct 18;23(1):236. doi: 10.1186/s12874-023-02049-6. BMC Med Res Methodol. 2023. PMID: 37853343 Free PMC article. - Rationale and design for Healthy Hearts for Michigan (HH4M): A pragmatic single-arm hybrid effectiveness-implementation study.
Krefman AE, Ciolino JD, Kan AK, Maki B, McHugh M, Smith JD, Bannon J, Carroll AJ, Bustamante P, Frye C, Hitsman B, Day A, Walunas TL. Krefman AE, et al. Contemp Clin Trials Commun. 2023 Aug 21;35:101199. doi: 10.1016/j.conctc.2023.101199. eCollection 2023 Oct. Contemp Clin Trials Commun. 2023. PMID: 37671245 Free PMC article. - Impact of Sources of Strength on adolescent suicide deaths across three randomized trials.
Wyman P, Cero I, Brown CH, Espelage D, Pisani A, Kuehl T, Schmeelk-Cone K. Wyman P, et al. Inj Prev. 2023 Oct;29(5):442-445. doi: 10.1136/ip-2023-044944. Epub 2023 Jul 28. Inj Prev. 2023. PMID: 37507212 Free PMC article. - Whose knowledge counts? Involving communities in intervention and trial design using community conversations.
Burgess RA, Shittu F, Iuliano A, Haruna I, Valentine P, Bakare AA, Colbourn T, Graham HR, McCollum ED, Falade AG, King C; INSPIRING Project Consortium. Burgess RA, et al. Trials. 2023 Jun 7;24(1):385. doi: 10.1186/s13063-023-07320-1. Trials. 2023. PMID: 37287035 Free PMC article. Clinical Trial. - Expanding population-level interventions to help more low-income smokers quit: Study protocol for a randomized controlled trial.
Wolff JM, McQueen A, Garg R, Thompson T, Fu Q, Brown DS, Kegler M, Carpenter KM, Kreuter MW. Wolff JM, et al. Contemp Clin Trials. 2023 Jun;129:107202. doi: 10.1016/j.cct.2023.107202. Epub 2023 Apr 18. Contemp Clin Trials. 2023. PMID: 37080354 Free PMC article.
References
- Angrist JD, Imbens GW, Rubin DB. Identification of causal effects using instrumental variables. J. Am. Stat. Assoc. 1996;91:444–55.
- Ayanlowo A, Redden D. A two-stage conditional power adaptive design adjusting for treatment by covariate interaction. Contemp. Clin. Trials. 2008;29:428–38. - PubMed
- Barnard J, Frangakis CE, Hill JL, Rubin DB. Principal stratification approach to broken randomized experiments: a case study of school choice vouchers in New York City/Comment/Rejoinder. J. Am. Stat. Assoc. 2003;98:299–323.
- Bartlett RH, Gazzaniga AB, Jefferies MR, Huxtable RF, Haiduc NJ, Fong SW. Extracorporeal membrane oxygenation (ECMO) cardiopulmonary support in infancy. Trans. Am. Soc. Artif. Intern. Organs. 1976;22:80–93. - PubMed
- Bartlett RH, Roloff DW, Cornell RG, Andrews AF, Dillon PW, Zwischenberger JB. Extracorporeal circulation in neonatal respiratory failure: a prospective randomized study. Pediatrics. 1985;76:479–87. - PubMed
Publication types
MeSH terms
Grants and funding
- P20 MH071897/MH/NIMH NIH HHS/United States
- R01 MH068423/MH/NIMH NIH HHS/United States
- R01MH068423/MH/NIMH NIH HHS/United States
- R34MH071189/MH/NIMH NIH HHS/United States
- R01 DA025192/DA/NIDA NIH HHS/United States
- R01MH069353/MH/NIMH NIH HHS/United States
- R01 MH078016-02/MH/NIMH NIH HHS/United States
- P30 DA023920-02/DA/NIDA NIH HHS/United States
- P30 DA023920/DA/NIDA NIH HHS/United States
- R01 MH066319/MH/NIMH NIH HHS/United States
- P30 MH068685/MH/NIMH NIH HHS/United States
- R01 MH076158-03/MH/NIMH NIH HHS/United States
- R01 MH040859-20/MH/NIMH NIH HHS/United States
- R01 MH069353-03/MH/NIMH NIH HHS/United States
- R01 DA025192-01A1/DA/NIDA NIH HHS/United States
- R01 MH069353/MH/NIMH NIH HHS/United States
- P30MH068685/MH/NIMH NIH HHS/United States
- P20 MH071897-05/MH/NIMH NIH HHS/United States
- R01 MH040859/MH/NIMH NIH HHS/United States
- R34 MH071189/MH/NIMH NIH HHS/United States
- P20MH071897/MH/NIMH NIH HHS/United States
- R01MH080122/MH/NIMH NIH HHS/United States
- R01MH066319/MH/NIMH NIH HHS/United States
- R01 MH068423-05/MH/NIMH NIH HHS/United States
- R01 MH080122-02/MH/NIMH NIH HHS/United States
- R01 MH066319-05/MH/NIMH NIH HHS/United States
- R34 MH071189-03/MH/NIMH NIH HHS/United States
- R01 MH076158/MH/NIMH NIH HHS/United States
- R01DA 025192/DA/NIDA NIH HHS/United States
- R01 MH078016/MH/NIMH NIH HHS/United States
- R01MH076158/MH/NIMH NIH HHS/United States
- SM57405/SM/CMHS SAMHSA HHS/United States
- P30 MH074678/MH/NIMH NIH HHS/United States
- R01-MH40859/MH/NIMH NIH HHS/United States
- R01 MH080122/MH/NIMH NIH HHS/United States
- R01MH078016/MH/NIMH NIH HHS/United States
- R01MH06624/MH/NIMH NIH HHS/United States
- P30 MH074678-02/MH/NIMH NIH HHS/United States
- U54 NS057405-01A19002/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources