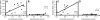Elevated corticosterone associated with food deprivation upregulates expression in rat skeletal muscle of the mTORC1 repressor, REDD1 - PubMed (original) (raw)
Elevated corticosterone associated with food deprivation upregulates expression in rat skeletal muscle of the mTORC1 repressor, REDD1
Nora K McGhee et al. J Nutr. 2009 May.
Abstract
Food deprivation induces a repression of protein synthesis in skeletal muscle in part due to reduced signaling through the mammalian target of rapamycin complex 1 (mTORC1). Previous studies have identified upregulated expression of the protein Regulated in DNA Damage and Development (REDD1) as an important mechanism in the regulation of mTORC1 activity in response to a variety of stresses. Our goal in this investigation was to determine whether modulation of REDD1 expression occurs in response to food deprivation and refeeding, and, if it does, to ascertain if changes in REDD1 expression correlate with altered mTORC1 signaling. As expected, mTORC1 signaling was repressed after 18 h of food deprivation compared with freely-fed control rats and quickly recovered after refeeding for 45 min. Food deprivation caused a dramatic rise in REDD1 mRNA and protein expression; refeeding resulted in a reduction to baseline. Food deprivation is characterized by low-serum insulin and elevated glucocorticoid concentrations. Therefore, initially, alloxan-induced type I diabetes was used to minimize the food deprivation- and refeeding-induced changes in insulin. Although diabetic rats exhibited upregulated REDD1 expression compared with nondiabetic controls, there was no direct correlation between REDD1 mRNA expression and serum insulin levels, and insulin treatment of diabetic rats did not affect REDD1 expression. In contrast, serum corticosterone levels correlated directly with REDD1 mRNA expression (r = 0.68; P = 0.01). Moreover, inhibiting corticosterone-mediated signaling via administration of the glucocorticoid receptor antagonist RU486 blocked both the food deprivation- and diabetes-induced increase in REDD1 mRNA expression. Overall, the results demonstrate that changes in REDD1 expression likely contribute to the regulation of mTORC1 signaling during food deprivation and refeeding.
Figures
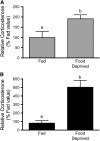
FIGURE 1
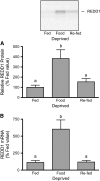
REDD1 protein (A) and mRNA expression (B) in gastrocnemius muscle of rats that consumed food ad libitum (Fed), were food deprived for 18 h, or were refed for 45 min after an 18-h deprivation (Re-fed). Values are means ± SEM, n ± 6. Means without a common letter differ, P < 0.05.
FIGURE 2
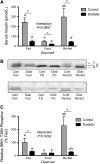
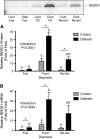
Serum insulin level (A), S6K1 hyperphosphorylation (B), and S6K1 phosphorylation on Thr389 (C) in gastrocnemius muscle of control (gray bars) and diabetic (black bars) rats that consumed food ad libitum (Fed), were food deprived for 18 h, or were refed for 45 min after an 18-h deprivation (Re-fed). Values are means ± SEM, n = 5–6. Within diabetic and control groups, means without a common letter differ, P < 0.05. *Different from corresponding control, P < 0.05.
FIGURE 3
REDD1 protein (A) and mRNA expression (B) in gastrocnemius muscle of control (gray bars) and diabetic (black bars) rats that consumed food ad libitum (Fed), were food deprived for 18 h, or were refed for 45 min after an 18-h deprivation (Re-fed). Values are means ± SEM, n = 6. Within diabetic and control groups, means without a common letter differ, P < 0.05. *Different from corresponding control, P < 0.05.
FIGURE 4
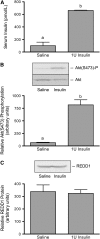
Serum insulin concentration (A), Akt(Ser473) phosphorylation (B), and REDD1 expression (C) in gastrocnemius muscle of control and insulin-treated diabetic rats. Values are means ± SEM, n = 6. Means without a common letter differ, P < 0.05.
FIGURE 5
Serum corticosterone concentrations in fed and food-deprived control (A) or diabetic (B) rats. (B) Serum corticosterone concentrations were measured in serum from fed and food-deprived diabetic rats. Values are means ± SEM, n = 6. Means without a common letter differ, P < 0.05.
FIGURE 6
Serum corticosterone concentration is directly proportional to REDD1 mRNA expression in gastrocnemius of control (A) and diabetic (C) rats administered vehicle but not in control (B) or diabetic (D) rats administered RU486.
Similar articles
- Rapid induction of REDD1 expression by endurance exercise in rat skeletal muscle.
Murakami T, Hasegawa K, Yoshinaga M. Murakami T, et al. Biochem Biophys Res Commun. 2011 Feb 25;405(4):615-9. doi: 10.1016/j.bbrc.2011.01.078. Epub 2011 Jan 25. Biochem Biophys Res Commun. 2011. PMID: 21272563 - Regulated in DNA damage and development 1 (REDD1) promotes cell survival during serum deprivation by sustaining repression of signaling through the mechanistic target of rapamycin in complex 1 (mTORC1).
Dennis MD, McGhee NK, Jefferson LS, Kimball SR. Dennis MD, et al. Cell Signal. 2013 Dec;25(12):2709-16. doi: 10.1016/j.cellsig.2013.08.038. Epub 2013 Sep 7. Cell Signal. 2013. PMID: 24018049 Free PMC article. - Nutrient-induced stimulation of protein synthesis in mouse skeletal muscle is limited by the mTORC1 repressor REDD1.
Gordon BS, Williamson DL, Lang CH, Jefferson LS, Kimball SR. Gordon BS, et al. J Nutr. 2015 Apr;145(4):708-13. doi: 10.3945/jn.114.207621. Epub 2015 Feb 25. J Nutr. 2015. PMID: 25716553 Free PMC article. - Emerging role for regulated in development and DNA damage 1 (REDD1) in the regulation of skeletal muscle metabolism.
Gordon BS, Steiner JL, Williamson DL, Lang CH, Kimball SR. Gordon BS, et al. Am J Physiol Endocrinol Metab. 2016 Jul 1;311(1):E157-74. doi: 10.1152/ajpendo.00059.2016. Epub 2016 May 17. Am J Physiol Endocrinol Metab. 2016. PMID: 27189933 Free PMC article. Review. - Is REDD1 a metabolic double agent? Lessons from physiology and pathology.
Britto FA, Dumas K, Giorgetti-Peraldi S, Ollendorff V, Favier FB. Britto FA, et al. Am J Physiol Cell Physiol. 2020 Nov 1;319(5):C807-C824. doi: 10.1152/ajpcell.00340.2020. Epub 2020 Sep 2. Am J Physiol Cell Physiol. 2020. PMID: 32877205 Review.
Cited by
- Multifaceted role of insulin-like growth factors and mammalian target of rapamycin in skeletal muscle.
Frost RA, Lang CH. Frost RA, et al. Endocrinol Metab Clin North Am. 2012 Jun;41(2):297-322, vi. doi: 10.1016/j.ecl.2012.04.012. Epub 2012 May 10. Endocrinol Metab Clin North Am. 2012. PMID: 22682632 Free PMC article. Review. - Control of translation initiation through integration of signals generated by hormones, nutrients, and exercise.
Kimball SR, Jefferson LS. Kimball SR, et al. J Biol Chem. 2010 Sep 17;285(38):29027-32. doi: 10.1074/jbc.R110.137208. Epub 2010 Jun 24. J Biol Chem. 2010. PMID: 20576612 Free PMC article. Review. - Methylseleninic acid elevates REDD1 and inhibits prostate cancer cell growth despite AKT activation and mTOR dysregulation in hypoxia.
Sinha I, Allen JE, Pinto JT, Sinha R. Sinha I, et al. Cancer Med. 2014 Apr;3(2):252-64. doi: 10.1002/cam4.198. Epub 2014 Feb 7. Cancer Med. 2014. PMID: 24515947 Free PMC article. - An oat bran meal influences blood insulin levels and related gene sets in peripheral blood mononuclear cells of healthy subjects.
Ulmius M, Johansson-Persson A, Krogh M, Olsson P, Onning G. Ulmius M, et al. Genes Nutr. 2011 Nov;6(4):429-39. doi: 10.1007/s12263-011-0236-8. Epub 2011 May 19. Genes Nutr. 2011. PMID: 21594609 Free PMC article. - DNA Damage-Inducible Transcript 4 Is an Innate Surveillant of Hair Follicular Stress in Vitamin D Receptor Knockout Mice and a Regulator of Wound Re-Epithelialization.
Zhao H, Rieger S, Abe K, Hewison M, Lisse TS. Zhao H, et al. Int J Mol Sci. 2016 Nov 26;17(12):1984. doi: 10.3390/ijms17121984. Int J Mol Sci. 2016. PMID: 27898044 Free PMC article.
References
- Li JB, Higgins JE, Jefferson LS. Changes in protein turnover in skeletal muscle in response to fasting. Am J Physiol. 1979;236:E222–8. - PubMed
- Rhoads RE. Signal transduction pathways that regulate eukaryotic protein synthesis. J Biol Chem. 1999;274:30337–40. - PubMed
- Kimball SR. The role of nutrition in stimulating muscle protein accretion at the molecular level. Biochem Soc Trans. 2007;35:1298–301. - PubMed
- Richardson CJ, Schalm SS, Blenis J. PI3-kinase and TOR: PIKTORing cell growth. Semin Cell Dev Biol. 2004;15:147–59. - PubMed
- Marcotrigiano J, Gingras AC, Sonenberg N, Burley SK. Cap-dependent translation initiation in eukaryotes is regulated by a molecular mimic of eIF4G. Mol Cell. 1999;3:707–16. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials