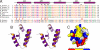Assembling stable hair cell tip link complex via multidentate interactions between harmonin and cadherin 23 - PubMed (original) (raw)
Assembling stable hair cell tip link complex via multidentate interactions between harmonin and cadherin 23
Lifeng Pan et al. Proc Natl Acad Sci U S A. 2009.
Abstract
The hereditary hearing-vision loss disease Usher syndrome (USH) is caused by defects in several proteins, most of which form an integrated protein network called Usher interactome. Harmonin/Ush1C is a master scaffold in the assembly of the Usher protein complexes, because harmonin is known to bind to every protein in the Usher interactome. However, the biochemical and structural mechanism governing the Usher protein complex formation is largely unclear. Here, we report that the highly-conserved N-terminal fragment of harmonin (N-domain) immediately preceding its PDZ1 adopts an autonomously-folded domain. We discovered that the N-domain specifically binds to a short internal peptide fragment of the cadherin 23 cytoplasmic domain. The structures of the harmonin N-domain alone and in complex with the cadherin 23 internal peptide fragment uncovered the detailed binding mechanism of this interaction between harmonin and cadherin 23. We further elucidated the harmonin PDZ domain-mediated cadherin 23 binding by solving the structure of the second harmonin PDZ domain in complex with the cadherin 23 carboxyl tail. The multidentate binding mode between harmonin and cadherin 23 provides a structural and biochemical basis for the harmonin-mediated assembly of stable tip link complex in the auditory hair cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Harmonin contains a novel N-terminal domain. (A) Structure-based sequence alignment of the N-domain from the different harmonin family members. In this alignment, the conserved hydrophobic residues are shown in orange, negatively-charged residues are in magenta, positively-charged residues are in blue, and the rest of the highly-conserved residues are in green. The residues that are critical for binding to the cadherin 23 peptide are boxed and highlighted by red stars or triangles. For comparison, the N-terminal region of whirlin from human and mouse are also included. (B) Stereoview of a representative NMR structure of the harmonin N-domain drawn in the ribbon diagram. (C) Surface representation showing the existence of a unique exposed hydrophobic pocket in the N-domain (highlighted by a black circle), which is hypothesized to serve as the binding site for potential target proteins. In this presentation, the hydrophobic amino acid residues are drawn in yellow, the positively-charged residues are blue, the negatively-charged residues are red, and the uncharged polar residues are gray.
Fig. 2.
Harmonin N-domain binds to the cytoplasmic domain of cadherin 23. (A) Western blot analysis showing that GST-fused harmonin N-domain binds to the cytoplasmic domain of cadherin 23. Lane 1 represents cadherin 23 input. (B) Superposition plot of the 1H-15N HSQC spectrum of the free N-domain and the protein in the presence of a saturating amount of GB1-tagged cadherin 23 peptide. For clarity, only a selected region of each spectrum is shown. The chemical shift assignments for the free- and cadherin 23 peptide bound-forms of the N-domain are labeled. (C) Amino acid sequence alignment of the 31-residue harmonin N-domain-binding fragment of cadherin 23. The crucial residues involved in binding to the harmonin N-domain are highlighted by red stars. The minimal 20-residue harmonin N-domain binding fragment of cadherin 23 are boxed. (D) Fluorescence-based measurement of the binding affinity of the harmonin N-domain toward the cadherin 23 peptide. (E) Overlay plot of the 1H-15N HSQC spectra of the free N-domain and the protein fused with a cadherin 23 (A3190E) peptide at its C terminus.
Fig. 3.
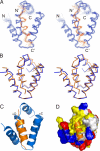
The structure of the N-domain and the Cad23 peptide complex. (A) Stereoview showing the backbones of 20 superimposed NMR structures of the harmonin N-domain in complex with the Cad23 peptide. The Cad23 peptides are drawn in orange. (B) Stereoview showing the comparison of the conformations of the free N-domain (blue) and the N-domain in complex with the Cad23 peptide (orange). (C) Ribbon diagram of a representative NMR structure of the N-domain/Cad23 peptide complex. (D) Surface representation of the harmonin N-domain/Cad23 peptide complex. N-domain is shown in surface representation using the same coloring scheme as in Fig. 1_C_, and the Cad23 peptide is drawn in the ribbon model.
Fig. 4.
Molecular details of the interaction between the N-domain and the Cad23 peptide. (A) Stereoview showing the detailed interactions of the Cad23 peptide with the residues from the harmonin N-domain. (B) Western blot analysis showing the interactions between cadherin 23 with 2 harmonin N-domain mutants. The figure also shows that the corresponding N-domain from whirlin does not bind to cadherin 23.
Fig. 5.
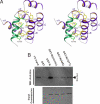
The multidentate interaction between harmonin and cadherin 23. (A) Specific interaction between harmonin PDZ2 and Cad23–PBM revealed by the NMR structure of the PDZ domain/peptide complex. Cad23-PBM is shown in red. The boxed region represents the Cad23–PBM-binding groove of the PDZ domain, and the detailed interactions between Cad23–PBM and the PDZ2 domain are drawn in the explicit atomic model. (Bi–Eiii) Both the harmonin N-domain/cadherin 23 internal peptide and the PDZ2/Cad23–PBM interactions are required for colocalization of harmonin and cadherin 23 in HeLa cells. (Bi–Biii) IL2R and harmonin did not colocalize with each other when the 2 proteins were overexpressed in HeLa cells. (Ci–Ciii) IL2R-cadherin 23 cytoplasmic domain chimera and harmonin colocalized with each other in the edges of cells (arrows). (Di–Eiii) Deletion of either the N-domain (Di–Diii) or PDZ2 (Ei–Eiii) of harmonin compromised the colocalization of harmonin and cadherin 23 in HeLa cells. (F) A schematic diagram depicting a model showing the synergistic, multidentate interaction between dimerized cadherin 23 and polyvalent harmonin in the tip link of hair cells. In this mode, harmonin via its N-domain and PDZ2 interacts with an internal peptide fragment and the C-terminal PBM of the cytoplasmic domain of cadherin 23. This multidendate interaction between harmonin and cadherin 23, together with the dimerization of cadherin 23, greatly enhances both specificity and stability of the assembly of the tip link complex in hair cells. Harmonin can further connect the tip link complex to the actin cytoskeleton via its PST module.
References
- Reiners J, Wolfrum U. Molecular analysis of the supramolecular usher protein complex in the retina. Harmonin as the key protein of the Usher syndrome. Adv Exp Med Biol. 2006;572:349–353. -PubMed
- El-Amraoui A, Petit C. Usher I syndrome: Unraveling the mechanisms that underlie the cohesion of the growing hair bundle in inner ear sensory cells. J Cell Sci. 2005;118:4593–4603. -PubMed
- Ahmed ZM, Riazuddin S, Riazuddin S, Wilcox ER. The molecular genetics of Usher syndrome. Clin Genet. 2003;63:431–444. -PubMed
- Ebermann I, et al. A novel gene for Usher syndrome type 2: Mutations in the long isoform of whirlin are associated with retinitis pigmentosa and sensorineural hearing loss. Hum Genet. 2007;121:203–211. -PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases