Inflammation-induced, 3'UTR-dependent translational inhibition of Hsp70 mRNA impairs intestinal homeostasis - PubMed (original) (raw)
Inflammation-induced, 3'UTR-dependent translational inhibition of Hsp70 mRNA impairs intestinal homeostasis
Shien Hu et al. Am J Physiol Gastrointest Liver Physiol. 2009 May.
Abstract
Although the inducible heat shock protein 70 (Hsp70) is essential for maintaining intestinal homeostasis in colitis, it is translationally downregulated in inflamed colonic mucosa, paradoxically rendering the gut more susceptible to injury. We examined the basis for this process by analyzing the role of untranslated regions (UTR) of Hsp70 mRNA in inflammation-associated downregulation in vitro and in vivo. Using luciferase-reporter assays in young adult mouse intestinal epithelial cells, we determined that cytokine-induced translational inhibition of Hsp70 mRNA was mediated by the 3'UTR, but not 5'UTR. In vivo, dextran sodium sulfate (DSS) colitis was induced in wild-type (WT) and villin-promoter regulated "UTR-less" Hsp70 transgenic (TG) mice, the latter exhibiting intestinal epithelial-specific transgene expression. Progressive downregulation of colonic Hsp70 protein expression was observed in WT, but not in TG, mice with increasing severity of mucosal inflammation, confirming the essential role of the 3'UTR in mediating inflammation-associated downregulation of Hsp70. Hsp70 TG mice demonstrated significantly lower endoscopic and histological inflammation scores in DSS-induced colitis than WT. In conclusion, downregulation of Hsp70 expression in inflamed mucosa is mediated by translational inhibition requiring the 3'UTR, resulting in increased mucosal injury. By forcing intestinal epithelial-specific Hsp70 expression in vivo, the severity of experimentally induced colitis was significantly reduced.
Figures
Fig. 1.
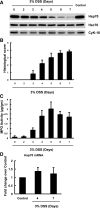
Colonic heat shock protein 70 (Hsp70) decreases in dextran sodium sulfate (DSS)-induced inflammation. C57BL/6 mice were given 3% wt/vol DSS for up to 7 days in their drinking water. On days 0, 2, 3, 4, 5, 6, and 7, DSS-treated and control mice were euthanized. Mucosa from transverse colon were harvested for the following: Western blot of Hsp70, Hsc70 and cytokeratin-18 (A); histological grading of intestinal inflammation (B); and myeloperoxidase (MPO) activity (μg/g) (C). D: real-time PCR analysis of mRNA abundance of Hsp70 in control and DSS-treated mouse colonic mucosa on days 4 and 7. Changes in Hsp mRNA relative to GAPDH were determined by 2−ΔΔCt as fold change over control. Results are means ± SE; n = 4.
Fig. 2.
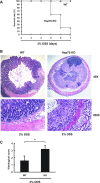
Hsp70 knockout (KO) mice are more susceptible to DSS treatment than wild-type (WT) mice. Hsp70 KO mice (Hsp 70.1−/− and 70.3−/−) and WT littermates were given DSS in drinking water. A: survival rate of WT and Hsp70 KO mice given 3% DSS for 7 days. B: WT and Hsp70 KO mice were treated with 2% DSS for 5 days and euthanized 5 days after treatment. The transverse colon was harvested for hematoxylin and eosin staining. Images shown are representative of 5 individual experiments. C: histological grading of intestinal inflammation in WT and Hsp70 KO mice treated with 2% DSS. *P < 0.05 compared WT and KO mice; n = 5.
Fig. 3.
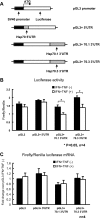
Hsp70 3′ untranslated regions (UTRs) participate in translational inhibition induced by IFN-γ and TNF-α in young adult mouse colonic epithelial cells (YAMC) cells. A: schematic of constructs expressing chimeric luciferase transcripts containing 5′- or 3′UTR of Hsp70. All constructs derived from pGL3-promoter contain the Firefly luciferase-coding region as a reporter gene and full-length 5′UTR or 3′UTR of mouse Hsp 70.1 and 70.3. Mouse Hsp 70.1 and 70.3 mRNAs have identical 5′UTR nucleotide sequences. Transcription of the luciferase-coding region is under the control of the SV40 promoter. Luciferase vectors, pGL3 and pRL-TK, were transfected into YAMC cells. 16 h later, cells were treated with IFN-γ (200 U/ml) and TNF-α (100 ng/ml) for 8 h and then subjected to heat shock. B: firefly luciferase activity normalized with Renilla luciferase activity. C: firefly luciferase mRNA was analyzed by real-time PCR and normalized to Renilla luciferase mRNA as the transfection efficiency control. Results are means ± SE; n = 4. *P < 0.05 compared IFN + TNF(−) and IFN + TNF(+) by _t_-test.
Fig. 4.
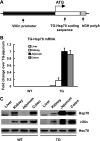
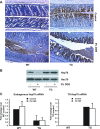
Intestine-specific Hsp70 expression from “UTR-less” Hsp70 mRNA in villin-Hsp70 transgenic (TG) mice. A: schematic of targeting transgene construct. The 11.1-kbp fragment, comprising the villin promoter followed by the human Hsp70 protein coding sequence and bovine growth hormone polyA signal, was used to generate TG mice on C57BL/6 background. B: expression of TG Hsp70 mRNA (TG-Hsp70) in liver, kidney, jejunum, and transverse colon of WT and TG mice. C: Hsp70, villin, and Hsc70 were analyzed by Western blot. Images shown are representative of 4 individual experiments.
Fig. 5.
Protein expression of UTR-less Hsp70 mRNA is resistant to DSS-induced inflammation. WT and Hsp70 UTR-less TG mice were given 3% DSS for 7 days and then euthanized. Mice given normal tap water were euthanized as controls. Colonic mucosa of transverse colon was harvested for the following: immunohistochemistry staining of Hsp70 (A), Western blot of Hsp70 and Hsc70 (B), and real-time PCR of endogenous Hsp70 mRNA and UTR-less TG-Hsp70 mRNA (C).
Fig. 6.
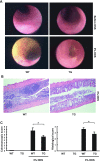
Endoscopic and histological grading of DSS colitis in WT and Hsp70 TG mice. WT and Hsp70 UTR-less TG mice were given 3% DSS for 7 days and then given tap water for another 3 days before analysis. A: colonoscopy was performed before and after DSS treatment in WT and TG mice under anesthesia. An endoscopic score was calculated. Images shown are representative of 4 sets of experiments. B: after colonoscopy, WT and TG mice were euthanized for hematoxylin and eosin staining and histological grading of colitis. C: endoscopic score and histological score of various mouse groups. *P < 0.05 compared WT and TG mice after DSS treatment; n = 4.
References
- Arvans DL, Vavricka SR, Ren H, Musch MW, Kang L, Rocha FG, Lucioni A, Turner JR, Alverdy J, Chang EB. Luminal bacterial flora determines physiological expression of intestinal epithelial cytoprotective heat shock proteins 25 and 72. Am J Physiol Gastrointest Liver Physiol 288: G696–G704, 2005. - PubMed
- Bendz H, Ruhland SC, Pandya MJ, Hainzl O, Riegelsberger S, Brauchle C, Mayer MP, Buchner J, Issels RD, Noessner E. Human heat shock protein 70 enhances tumor antigen presentation through complex formation and intracellular antigen delivery without innate immune signaling. J Biol Chem 282: 31688–31702, 2007. - PubMed
- Burress GC, Musch MW, Jurivich DA, Welk J, Chang EB. Effects of mesalamine on the hsp72 stress response in rat IEC-18 intestinal epithelial cells. Gastroenterology 113: 1474–1479, 1997. - PubMed
- Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest 69: 238–249, 1993. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous