gamma-Secretase heterogeneity in the Aph1 subunit: relevance for Alzheimer's disease - PubMed (original) (raw)
. 2009 May 1;324(5927):639-42.
doi: 10.1126/science.1171176. Epub 2009 Mar 19.
Jérôme Van Biervliet, Katleen Craessaerts, Tim Dejaegere, Katrien Horré, Tine Van Houtvin, Hermann Esselmann, Sabine Paul, Martin K Schäfer, Oksana Berezovska, Bradley T Hyman, Ben Sprangers, Raf Sciot, Lieve Moons, Mathias Jucker, Zhixiang Yang, Patrick C May, Eric Karran, Jens Wiltfang, Rudi D'Hooge, Bart De Strooper
Affiliations
- PMID: 19299585
- PMCID: PMC2740474
- DOI: 10.1126/science.1171176
gamma-Secretase heterogeneity in the Aph1 subunit: relevance for Alzheimer's disease
Lutgarde Serneels et al. Science. 2009.
Abstract
The gamma-secretase complex plays a role in Alzheimer's disease and cancer progression. The development of clinically useful inhibitors, however, is complicated by the role of the gamma-secretase complex in regulated intramembrane proteolysis of Notch and other essential proteins. Different gamma-secretase complexes containing different Presenilin or Aph1 protein subunits are present in various tissues. Here we show that these complexes have heterogeneous biochemical and physiological properties. Specific inactivation of the Aph1B gamma-secretase in a mouse Alzheimer's disease model led to improvements of Alzheimer's disease-relevant phenotypic features without any Notch-related side effects. The Aph1B complex contributes to total gamma-secretase activity in the human brain, and thus specific targeting of Aph1B-containing gamma-secretase complexes may help generate less toxic therapies for Alzheimer's disease.
Figures
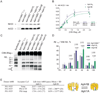
Fig. 1
Aph1B γ-secretase complexes are functional and structurally distinct relative to the Aph1A γ-secretase complexes. (A,B) Immunoblot analysis of microsomal fractions of Aph1ABC+/+ and Aph1ABC−/− MEF rescued with the indicated Aph1 isoforms demonstrates that both complexes perform ε-cleavage of APP and Notch substrate. Specificity of the reaction was validated using a γ-secretase inhibitor (γ−: 10µM L L-685,458). (B) The velocity, or amount of AICD generated after 3 h, was plotted against C99-3flag concentrations. The kinetics of AICD fit a Michaelis-Menten with positive cooperation (h=2) equation. Apparent Vmax and KM values for AICD were statistically indistinguishable (mean±SD, N=5). (C, D) Urea-SDS-PAGE of solubilised Aph1B γ-secretase complexes from MEFs results in more long (Aβ1≥42) and less short (Aβ1–≤40) Aβ species (Mean±SD, N=3, p<0.05-0.01). (E) Aph1ABC−/− MEFs reconstituted with Aph1AL or Aph1B were co-transfected with wild-type human PS1 cDNA and Alexa488 lifetimes were measured in absence or presence of FRET; Aph1B γ-secretase display a significant shorter life time (mean±SD, number of cells counted in 3 to 4 independent transfections are indicated between brackets, p<0.01) implying a closer proximity between the donor and acceptor as schematically represented.
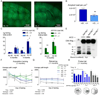
Fig. 2
Deletion of Aph1BC abolishes age-dependent rise in Aβ levels in the brain and rescues learning and memory deficits. (A, B) Decreased amyloid burden was evident in APPPS1+/0;_Aph1BC_−/− mice at 9 months of age (representative sections shown, measured using quantitative stereology (N=5, p<0.01, absolute amyloid load per unit volume, p<0.01). (C, D) Using Aβx–40 and Aβx–42-specific ELISA, Aβ-levels in the hippocampus were measured and normalized to total protein content. Accumulation of Aβx–40 and Aβx–42 were rescued in APPPS1+/0 and APPPS1+/+ mice (N=5, p<0.05). (E) Purification of the γ-secretase complexes from APPPS1+/0;Aph1BC+/+ and APPPS1+/0;_Aph1BC_−/− brains using the same PS1-specific antibody, followed by an in vitro activity assay for AICD and Aβ production resulted in a quantitative decrease in APPPS1+/0;_Aph1BC_−/− mouse brain γ-secretase activity (56±1%) and a statistically significant qualitative shift in the pattern of Aβ produced (N=4, p<0.01). (F) At a young age (median 6.5 months; range 4.8–7.6), learning, as measured by decreasing path length, occurred in all genotypes, except APPPS1+/+;Aph1BC+/+ (Mean±SEM: genotype effect p<0.01; genotype×time interaction p<0.01). This deficit was significantly improved in the APPPS1+/+;_Aph1BC_−/− mice (p<0.05). (G) Retraining of the same mice at 11 months of age (median 10.7 months; range 9.2–12.2 months) showed that APPPS1+/0;Aph1BC+/+ mice were significantly worse than APPPS1+/0;Aph1BC−/− mice in finding the hidden platform during all retraining days (2-way RM ANOVA between APPPS1+/0 genotypes: genotype effect p<0.01). (H) Probe trial results confirm that Aph1BC deletion prevents spatial memory deficits in hemizygous APPPS1+/0;Aph1BC+/+ mice (%Time spent in: target quadrant TQ, adjacent quadrants AQ1–AQ2 and opposite quadrant OQ, p<0.05; vertical comparison bars: t-test with hypothetical mean 25% or chance level, p<0.001).
Fig. 3
Absence of Notch-signalling defects in _Aph1BC_−/− mice and expression of Aph1B/C in neurons of the adult mouse brain. (A, B, C) Sensitive T- and B-cell populations in the thymus (A, B) or in the spleen (C) were not distinguishable using FACS in age-matched Aph1BC+/+ (N=4) and _Aph1BC_−/− mice (N=3). (D) CD4+/CD8+ ratios of peripheral T-lymphocytes were identical in both _APPPS1_0/0 (_Aph1BC_−/−: N=3; Aph1BC+/+: N=4) and APPPS1+/0 mice (_Aph1BC_−/−: N=8; Aph1BC+/+: N=7). (E) Intestinal mucosal and (F) pancreas morphology, evaluated in a blind study, was identical in both genotypes (N=3; H&E and PAS; representative sections). (G, H , J–L). High power dark-field micrographs of hippocampal region (CA1) hybridized with the indicated antisense probes demonstrate that Aph1B/C expression is restricted to the pyramidal cell layer of the CA1 region and neuronal cell layers of cerebral cortex (layer VI) (G). Notch1 is predominantly expressed in non-neuronal layers containing mainly glial cells (H). Aph1A is widely expressed in both neuronal- and glia-enriched regions (J). Notch1 expression is generally low and partially overlaps with the non-neuronal markers GFAP (astrocytes) (K) and C1q (microglia, perivascular macrophages) (L). Hippocampal and cortical layers are identified by bright field photography of section depicted in (I). VI: lamina six of parietal cortex, cc: corpus callosum, alv: alveus of the hippocampus (astrocytes), Or: Stratum oriens of the CA1 region, Py: contains typical pyramidal neurons, Rad: Stratum radiatum (glia), LMol: Stratum lacunosum molecolare (glia).
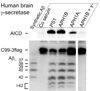
Fig. 4
Aph1B γ-secretase contributes to Aβ-production in human brain. Different pools of γ-secretase were prepared from microsomal membranes of human brain tissue using immunoprecipitation with pre-immune serum (Co serum), or PS1-, Aph1B-, or Aph1AL-specific antibody. γ-Secretase activity was then measured in vitro. Production of AICD and Aβ species was measured using SDS-PAGE or urea-SDS-PAGE and specific antibodies. Comparison of the Aph1AL and Aph1B precipitated pools reveals a statistically significant shift in the pattern of Aβ production (mean±SD, N=3, p<0.05-0.01). Notice that Aph1AL immunoprecipitate is less active and 4x more sample was loaded. γ−: 10µM γ-secretase inhibitor L-685,458
Comment in
- Medicine. Avoiding unintended toxicity.
Golde TE, Kukar TL. Golde TE, et al. Science. 2009 May 1;324(5927):603-4. doi: 10.1126/science.1174267. Science. 2009. PMID: 19407192 No abstract available.
Similar articles
- Specific combinations of presenilins and Aph1s affect the substrate specificity and activity of γ-secretase.
Yonemura Y, Futai E, Yagishita S, Kaether C, Ishiura S. Yonemura Y, et al. Biochem Biophys Res Commun. 2016 Sep 30;478(4):1751-7. doi: 10.1016/j.bbrc.2016.09.018. Epub 2016 Sep 5. Biochem Biophys Res Commun. 2016. PMID: 27608597 - Specific Mutations in Aph1 Cause γ-Secretase Activation.
Watanabe H, Yoshida C, Hidaka M, Ogawa T, Tomita T, Futai E. Watanabe H, et al. Int J Mol Sci. 2022 Jan 3;23(1):507. doi: 10.3390/ijms23010507. Int J Mol Sci. 2022. PMID: 35008932 Free PMC article. - Dominant negative effect of the loss-of-function γ-secretase mutants on the wild-type enzyme through heterooligomerization.
Zhou R, Yang G, Shi Y. Zhou R, et al. Proc Natl Acad Sci U S A. 2017 Nov 28;114(48):12731-12736. doi: 10.1073/pnas.1713605114. Epub 2017 Oct 9. Proc Natl Acad Sci U S A. 2017. PMID: 29078389 Free PMC article. - Toward the structure of presenilin/γ-secretase and presenilin homologs.
Wolfe MS. Wolfe MS. Biochim Biophys Acta. 2013 Dec;1828(12):2886-97. doi: 10.1016/j.bbamem.2013.04.015. Biochim Biophys Acta. 2013. PMID: 24099007 Free PMC article. Review. - Assembly of the presenilin γ-/ε-secretase complex.
St George-Hyslop P, Fraser PE. St George-Hyslop P, et al. J Neurochem. 2012 Jan;120 Suppl 1:84-88. doi: 10.1111/j.1471-4159.2011.07505.x. Epub 2011 Nov 28. J Neurochem. 2012. PMID: 22122073 Review.
Cited by
- Revisiting the Amyloid Cascade Hypothesis: From Anti-Aβ Therapeutics to Auspicious New Ways for Alzheimer's Disease.
Uddin MS, Kabir MT, Rahman MS, Behl T, Jeandet P, Ashraf GM, Najda A, Bin-Jumah MN, El-Seedi HR, Abdel-Daim MM. Uddin MS, et al. Int J Mol Sci. 2020 Aug 14;21(16):5858. doi: 10.3390/ijms21165858. Int J Mol Sci. 2020. PMID: 32824102 Free PMC article. Review. - Modulation of gamma-secretase reduces beta-amyloid deposition in a transgenic mouse model of Alzheimer's disease.
Kounnas MZ, Danks AM, Cheng S, Tyree C, Ackerman E, Zhang X, Ahn K, Nguyen P, Comer D, Mao L, Yu C, Pleynet D, Digregorio PJ, Velicelebi G, Stauderman KA, Comer WT, Mobley WC, Li YM, Sisodia SS, Tanzi RE, Wagner SL. Kounnas MZ, et al. Neuron. 2010 Sep 9;67(5):769-80. doi: 10.1016/j.neuron.2010.08.018. Neuron. 2010. PMID: 20826309 Free PMC article. - Expression Profiling of Notch Signalling Pathway and Gamma-Secretase Activity in the Brain of Ts1Cje Mouse Model of Down Syndrome.
Yusof HH, Lee HC, Seth EA, Wu X, Hewitt CA, Scott HS, Cheah PS, Li YM, Chau DM, Ling KH. Yusof HH, et al. J Mol Neurosci. 2019 Apr;67(4):632-642. doi: 10.1007/s12031-019-01275-2. Epub 2019 Feb 13. J Mol Neurosci. 2019. PMID: 30758748 Free PMC article. - Apo and Aβ46-bound γ-secretase structures provide insights into amyloid-β processing by the APH-1B isoform.
Odorčić I, Hamed MB, Lismont S, Chávez-Gutiérrez L, Efremov RG. Odorčić I, et al. Nat Commun. 2024 May 27;15(1):4479. doi: 10.1038/s41467-024-48776-2. Nat Commun. 2024. PMID: 38802343 Free PMC article. - Activation of ventral tegmental area vesicular GABA transporter (Vgat) neurons alleviates social defeat stress-induced anxiety in APP/PS1 mice.
Yao D, Li R, Kora M, Huang H, Liu X, Gong S. Yao D, et al. Front Aging Neurosci. 2023 Mar 23;15:1142055. doi: 10.3389/fnagi.2023.1142055. eCollection 2023. Front Aging Neurosci. 2023. PMID: 37032820 Free PMC article.
References
- De Strooper B, et al. Nature. 1998 Jan 22;391:387. - PubMed
- De Strooper B. Neuron. 2003 Apr 10;38:9. - PubMed
- Hebert SS, et al. Neurobiology of Disease. 2004 Nov 17;17:260. - PubMed
- Shirotani K, Edbauer D, Prokop S, Haass C, Steiner H. J. Biol. Chem. 2004 Oct 1;279:41340. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG026593/AG/NIA NIH HHS/United States
- P01 AG015379/AG/NIA NIH HHS/United States
- R01AG026593/AG/NIA NIH HHS/United States
- R01 AG026593-01A1/AG/NIA NIH HHS/United States
- AG026593/AG/NIA NIH HHS/United States
- AG 13579/AG/NIA NIH HHS/United States
- P01 AG015379-110009/AG/NIA NIH HHS/United States
- P01AG015379/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases