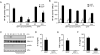Clathrin- and dynamin-dependent endocytic pathway regulates muramyl dipeptide internalization and NOD2 activation - PubMed (original) (raw)
Clathrin- and dynamin-dependent endocytic pathway regulates muramyl dipeptide internalization and NOD2 activation
Noemí Marina-García et al. J Immunol. 2009.
Abstract
Muramyl dipeptide (MDP), the NOD2 agonist, induces NF-kappaB and MAPK activation leading to the production of antimicrobial and proinflammatory molecules. MDP is internalized into acidified vesicles in macrophages. However, the endocytic mechanism of MDP uptake that induces NOD2 signaling is unknown. We now report the identification of an endocytosis pathway dependent on clathrin and dynamin that mediates MDP internalization and NOD2 activation. Intracellular MDP uptake was inhibited by chlorpromazine, a drug that disrupts clathrin-dependent endocytosis, but not by compounds that block pinocytosis or cellular entry via scavenger or mannose receptors. In contrast, MDP uptake and NOD2-dependent signaling were unimpaired in macrophages deficient in PepT1, a peptide transporter previously implicated in MDP internalization. Both chlorpromazine and knockdown of clathrin expression by RNA interference attenuated MDP-induced NF-kappaB and MAPK activation. Furthermore, MDP uptake and NOD2-dependent signaling were impaired by inhibition of dynamin, a GTPase required for budding of clathrin-coated vesicles from the plasma membrane. Finally, bafilomycin A, a specific inhibitor of the vacuolar proton pump, blocked MDP accumulation in acidified vesicles and cytokine responses, suggesting that vacuolar maturation is important for MDP-induced NOD2 signaling. These studies provide evidence for a clathrin- and dynamin-dependent endocytosis pathway that mediates MDP uptake and NOD2 activation.
Figures
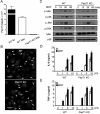
Figure 1. PepT1 is not required for fluorescent-labeled MDP uptake or MDP-induced signaling in mouse macrophages
(A) Expression of PepT1 mRNA isolated from bone marrow-derived macrophages (Mac) from WT and PepT1 KO mice by real-time PCR. Results were normalized to GAPDH levels. Expression of PepT1 in small intestine (SI) of WT mouse is shown for comparison. (B) Macrophages derived from WT and PepT1 KO mice were incubated for 3 h with MDP-Rhodamine (20 μg/ml, red), the nuclei were counterstained with DAPI (blue) and cells were fixed and imaged by confocal microscopy. Arrows indicate intracellular MDPRhodamine (C) Macrophages from WT and PepT1 KO were stimulated with MDP (10 μg/ml) for the indicated periods. Cell lysates were prepared and blotted with indicated antibodies. Results are from one representative experiment of three independent experiments. p, phosphorylated. (D, E) Macrophages from WT and PepT1 KO mice were stimulated with the indicated amount of LPS (ng/ml) in absence or presence of MDP (10 μg/ml). Cells supernatants were collected 24 h after stimulation and IL-6 and TNF-α were measured by ELISA. Results are presented as the mean of triplicate wells ± SD and correspond to one representative experiment of two independent experiments.
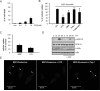
Figure 2. Effect of inhibitors of different cellular uptake pathways on the internalization of fluorescent-labeled MDP by mouse macrophages
(A) Bone-marrow derived macrophages were treated with the indicated doses of CPZ for 3. 5 hrs and the induction of cell death was evaluated by LDH release. Values represent mean ± SD of triplicate cultures. (B) Percentage of MDP-labeled macrophages after incubation for 15 min with medium alone (-), medium containing MDP-Alexa488 alone (untreated, unt.) or in the presence of CPZ (5 μM), dimethylamiloride (DMA), polyinosinic acid (Poly I) or mannans from Sacharomyces cerevesiae (Mannans). Results are representative of two experiments. (C) Bone-marrow derived macrophages were pretreated or not with CPZ (5 μM) for 30 minutes and then incubated with MDP-Alexa488 or LDL-BODIPY for 60 minutes. The uptake was analyzed by FACS and plotted as percent of uptake inhibition of the untreated cells. Results are presented as the mean of triplicate wells ± SD. Results are representative of two experiments (D) Bone-marrow derived macrophages were pretreated or not with CPZ (5 μM) and stimulated with MDP (10 μg/ml) for the indicated periods of time. Cell lysates were prepared and blotted with indicated antibodies. Results are from one representative experiment of three independent experiments. p, phosphorylated (E) Fluorescence confocal images of mouse macrophages incubated for 3 h with MDP-Rhodamine (red), alone or in the presence of CPZ or Poly I. In all cases, the nuclei were counterstained with DAPI (blue), and cells were fixed and imaged by fluorescence confocal microscopy. Arrows indicate intracellular MDP-Rhodamine *, denotes p < 0.05 between untreated and CPZ-treated cultures. Results are representative of three experiments.
Figure 3. Clathrin-mediated endocytosis regulates MDP-induced NOD2-dependent NF-κ B activation in HEK293T cells
(A) HEK293T cells were transiently transfected with a NF-κB luciferase reporter plasmid along with a control β-galactosidase plasmid (control), and NOD2 expression plasmid when indicated (NOD2), in triplicate. The cells were treated with medium alone or with medium containing 100 ng/ml MDP (MDP) for 16 h, in the absence (untreated) or presence of mannans, Poly I or CPZ. Luciferase and β-galactosidase activity was measured in cell lysates and values normalized for transfection efficiency (nRLU). (B) Luciferase reporter gene assays were performed as in (A). Transfected cells were treated with media alone (unst.) or with media containing either 100 ng/ml MDP or 10 ng/ml human TNF-α for 16 h, alone (untreated) or in the presence of CPZ. Results are presented as the mean of triplicate wells ± SD and correspond to one representative experiment of three independent experiments. *, p < 0.01 between untreated and CPZ-treated samples (Nod2+MDP). (C) HEK293T cells were transfected with a control plasmid (shRNA -) or two shRNA plasmids targeting the clathrin heavy chain gene (CHC) (shRNA1 and shRNA2). Cell lysates were prepared and blotted with indicated antibodies. (D, E) HEK293T cells were transiently transfected with a control plasmid (shRNA -) or with shRNA plasmids (shRNA1 and shRNA2). Luciferase reporter gene assays were performed as in A. Results are presented as the mean of triplicate wells ± SD and correspond to one representative experiment of three independent experiments. *, p < 0.05 between control shRNA- plasmid and shRNA1 or shRNA2 constructs in MDP stimulated cultures.
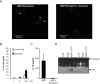
Figure 4. Dynasore impairs MDP-uptake and caspase-1 activation
(A) Fluorescence confocal images of mouse macrophages incubated for 3 h with MDP-Rhodamine (red), alone or in the presence of dynasore. In all cases, the nuclei were counterstained with DAPI (blue), and cells were fixed and imaged by fluorescence confocal microscopy. Arrows denote intracellular MDP-Rhodamine (B) Bone-marrow derived macrophages were treated with dynosore for the indicated time and the induction of cell death was evaluated by the release of LDH. Values represent mean ± SD of triplicate cultures. (C) Human monocytes were stimulated with 1 μg/ml MDP, in the absence (MDP) or in the presence of dynasore (MDP + Dynasore) for 16 hrs and the levels of IL-1β were measured in cell supernatants. Results are presented as the mean of triplicate wells ± SD and correspond to one representative experiment of three independent experiments. *, p < 0.05, statistically significant differences in the cytokines levels between untreated cells or dynasore-treated cells. (D) Bone-marrow derived macrophages were pretreated or not with dynasore and stimulated with MDP, ATP or MDP + ATP. Extracts were prepared from cell and culture supernatants and immunoblotted with caspase-1 antibody. Arrows denote procaspase-1 (procasp-1) and its processed p20 subunit. Results are representative of three independent experiment.
Figure 5. Fucntional dynamin is required for MDP-induced NOD2-mediated NF-kB activation
(A, B) Luciferase reporter gene assays were performed as in Figure 4. HEK293T cells transiently transfected in triplicate with a NF-κB luciferase reporter plasmid, along with a β-galactosidase transfection plasmid and a NOD2 expression plasmid (-), and together with 50 ng or 200 ng of a plasmid producing Dynamin (K44A), or with a control plasmid. The cells were treated with media alone (unst.), or media containing 100 ng/ml MDP, or 10 ng/ml human TNF-α for 16 h. Results are presented as the mean of triplicate wells ± SD and correspond to one representative experiment of three independent experiments. *, p < 0.05 between cells transfected with NOD2 plasmid and NOD2 plasmid + Dynamin (K44A) construct in MDP-stimulated cultures (C) Bone-marrow derived macrophages were pretreated or not with dynasore and stimulated with MDP (10 μg/ml) for the indicated periods of time. Cell lysates were prepared and blotted with indicated antibodies. p, phosphorylated. Results are from one representative experiment of three independent experiments. (D,E) Mouse macrophages were pretreated, or not, with dynasore and then stimulated, or not, with 10 μg/ml of MDP in the presence of 5 ng/ml of LPS. Cells supernatants were collected 24 h after stimulation and IL-6 and TNF-α were measured by ELISA. (F) Human monocytes were stimulated with 1 μg/ml MDP, in the absence (MDP) or in the presence of dynasore (MDP + Dynasore) for 16 hrs and the levels of IL-8 were measured in cell supernatants. Results are presented as the mean of triplicate wells ± SD and correspond to one representative experiment of three independent experiments. *, p < 0.05, statistically significant differences in the cytokines levels between untreated cells or dynasore-treated cells.
figure 6. Bafilomycin A blocks MDP-induced NF-κB activation in mouse macrophages and human monocytes
(A) Percentage of MDP-labeled macrophages after incubation for 15 min. with media alone (-), media containing MDP-Alexa488 (untreated, unt.) or MDP-Alexa488 in the presence of bafilomycin A (Baf. A). (B) Fluorescence confocal images of mouse macrophages incubated for 3 h with MDP-Rhodamine (red), alone or in the presence of Bafilomycin A. In all cases, the nuclei were counterstained with DAPI (blue), and cells were fixed and imaged by fluorescence confocal microscopy. Notice difference in size of intracellular MDP-Rhodamine structures between untreated and bafilomycin A-treated cells (C) Percentage of MDP-labeled cells that displayed granular coarse (large) pattern in the absence (MDP) or presence of bafilomycin A (MDP + Baf. A). The bars represent the mean of 15-20 optical sections ± SD and are representative of at least three different experiments. (D, E) Mouse macrophages were stimulated with 5 ng/ml of LPS alone (-) or together with 10 μg/ml of MDP, in the absence (MDP), or presence of bafilomycin A (MDP + Baf. A). Cells supernatants were collected 24 h after stimulation and IL-6 and TNF-α were measured by ELISA. (F) Human monocytes were stimulated with 1 μg/ml of MDP alone (MDP) or in the presence of bafilomycin A (MDP + Baf. A) for 16 hrs and levels of IL-8 were measured in cell supernatants. Results are presented as the mean of triplicate wells ± SD and correspond to one representative experiment of three independent experiments. *, p < 0.05 between MDP and MDP+bafilomycin A-treated cells.
Similar articles
- Pannexin-1-mediated intracellular delivery of muramyl dipeptide induces caspase-1 activation via cryopyrin/NLRP3 independently of Nod2.
Marina-García N, Franchi L, Kim YG, Miller D, McDonald C, Boons GJ, Núñez G. Marina-García N, et al. J Immunol. 2008 Mar 15;180(6):4050-7. doi: 10.4049/jimmunol.180.6.4050. J Immunol. 2008. PMID: 18322214 - pH-dependent internalization of muramyl peptides from early endosomes enables Nod1 and Nod2 signaling.
Lee J, Tattoli I, Wojtal KA, Vavricka SR, Philpott DJ, Girardin SE. Lee J, et al. J Biol Chem. 2009 Aug 28;284(35):23818-29. doi: 10.1074/jbc.M109.033670. Epub 2009 Jul 1. J Biol Chem. 2009. PMID: 19570976 Free PMC article. - Regulation of Clathrin-Mediated Endocytosis.
Mettlen M, Chen PH, Srinivasan S, Danuser G, Schmid SL. Mettlen M, et al. Annu Rev Biochem. 2018 Jun 20;87:871-896. doi: 10.1146/annurev-biochem-062917-012644. Epub 2018 Apr 16. Annu Rev Biochem. 2018. PMID: 29661000 Free PMC article. Review. - Freund's adjuvant, NOD2 and mycobacteria.
Behr MA, Divangahi M. Behr MA, et al. Curr Opin Microbiol. 2015 Feb;23:126-32. doi: 10.1016/j.mib.2014.11.015. Epub 2014 Dec 5. Curr Opin Microbiol. 2015. PMID: 25483349 Review.
Cited by
- NOD Signaling and Cell Death.
Heim VJ, Stafford CA, Nachbur U. Heim VJ, et al. Front Cell Dev Biol. 2019 Oct 2;7:208. doi: 10.3389/fcell.2019.00208. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31632962 Free PMC article. Review. - Genomics of lipid-laden human hepatocyte cultures enables drug target screening for the treatment of non-alcoholic fatty liver disease.
Breher-Esch S, Sahini N, Trincone A, Wallstab C, Borlak J. Breher-Esch S, et al. BMC Med Genomics. 2018 Dec 14;11(1):111. doi: 10.1186/s12920-018-0438-7. BMC Med Genomics. 2018. PMID: 30547786 Free PMC article. - Still finding ways to augment the existing management of acute and chronic kidney diseases with targeted gene and cell therapies: Opportunities and hurdles.
Corridon PR. Corridon PR. Front Med (Lausanne). 2023 Mar 7;10:1143028. doi: 10.3389/fmed.2023.1143028. eCollection 2023. Front Med (Lausanne). 2023. PMID: 36960337 Free PMC article. Review. - ATG16L1 and NOD2 interact in an autophagy-dependent antibacterial pathway implicated in Crohn's disease pathogenesis.
Homer CR, Richmond AL, Rebert NA, Achkar JP, McDonald C. Homer CR, et al. Gastroenterology. 2010 Nov;139(5):1630-41, 1641.e1-2. doi: 10.1053/j.gastro.2010.07.006. Epub 2010 Jul 14. Gastroenterology. 2010. PMID: 20637199 Free PMC article. - Regulation of the EGFR/ErbB signalling by clathrin in response to various ligands in hepatocellular carcinoma cell lines.
Liu Y, Calmel C, Desbois-Mouthon C, Sobczak-Thépot J, Karaiskou A, Praz F. Liu Y, et al. J Cell Mol Med. 2020 Jul;24(14):8091-8102. doi: 10.1111/jcmm.15440. Epub 2020 Jun 9. J Cell Mol Med. 2020. PMID: 32515546 Free PMC article.
References
- Inohara N, Chamaillard M, McDonald C, Nunez G. NOD-LRR proteins: role in host-microbial interactions and inflammatory disease. Annu Rev Biochem. 2005;74:355–383. - PubMed
- Franchi L, Park JH, Shaw MH, Marina-Garcia N, Chen G, Kim YG, Nunez G. Intracellular NOD-like receptors in innate immunity, infection and disease. Cell Microbiol. 2008;10:1–8. - PubMed
- Inohara N, Ogura Y, Fontalba A, Gutierrez O, Pons F, Crespo J, Fukase K, Inamura S, Kusumoto S, Hashimoto M, Foster SJ, Moran AP, Fernandez-Luna JL, Nunez G. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn's disease. J Biol Chem. 2003;278:5509–5512. - PubMed
- Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott DJ, Sansonetti PJ. NOD2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278:8869–8872. - PubMed
- Chin AI, Dempsey PW, Bruhn K, Miller JF, Xu Y, Cheng G. Involvement of receptor-interacting protein 2 in innate and adaptive immune responses. Nature. 2002;416:190–194. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GM065248/GM/NIGMS NIH HHS/United States
- R01 DK061707/DK/NIDDK NIH HHS/United States
- R01 DK067628/DK/NIDDK NIH HHS/United States
- R01 GM035498/GM/NIGMS NIH HHS/United States
- R01 GM065248/GM/NIGMS NIH HHS/United States
- GM035498/GM/NIGMS NIH HHS/United States
- DK61707/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources