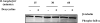The antibiotics doxycycline and minocycline inhibit the inflammatory responses to the Lyme disease spirochete Borrelia burgdorferi - PubMed (original) (raw)
The antibiotics doxycycline and minocycline inhibit the inflammatory responses to the Lyme disease spirochete Borrelia burgdorferi
Andrea L F Bernardino et al. J Infect Dis. 2009.
Abstract
Tetracyclines moderate inflammatory responses of various etiologies. We hypothesized that tetracyclines, in addition to their antimicrobial function, could exert control over the inflammation elicited by Borrelia burgdorferi. To model systemic effects, we used the human monocytic cell line THP-1; to model effects in the central nervous system, we used rhesus monkey brain astrocytes and microglia. Cells were stimulated with live or sonicated B. burgdorferi or with the lipoprotein outer surface protein A in the presence of increasing concentrations of doxycycline or minocycline. Both antibiotics significantly reduced the production of tumor necrosis factor-alpha, interleukin (IL)-6, and IL-8 in a dose-dependent manner in all cell types. Microarray analyses of the effect of doxycycline on gene transcription in spirochete-stimulated monocytes revealed that the NFKB and CHUK (alias, IKKA) genes were down-regulated. Functionally, phosphorylation of IkappaBalpha and binding of NF-kappaB to target DNA were both reduced in these cells. Our results suggest that tetracyclines may have a dual therapeutic effect in Lyme disease.
Figures
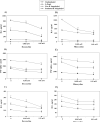
Figure 1. Doxycycline and minocycline affect the secretion of pro-inflammatory mediators by stimulated THP-1 cells
Concentrations of IL-6 (A, D), TNF-alpha (B, E), and IL-8 (C, F) in THP-1 cells were determined after incubation in medium alone or with 0.005 mM, or 0.01 mM of doxycycline (1) or minocycline (2) for 24 h, followed by incubations with fresh doxycycline at the above concentrations, and with 0.25 μg/ml L-OspA, live, sonicated B. burgdorferi (both at 10 MOI), or no stimulant. Shown are the mean values ± SD obtained from triplicate specimens. * (P<0.05) and ** (P<0.005) indicates significant differences between untreated and doxycycline/minocycline-treated cells in a dose-dependent manner. Similar results were obtained with supernatants from cells of 3 additional independent experiments.
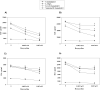
Figure 2. Doxycycline markedly reduces the secretion of pro-inflammatory mediators in stimulated primary glial cells
IL-6 (A, C) and IL-8 (B, D) production by microglia (1) and astrocytes (2) was measured after pre-incubation of the cells in medium alone or pre-treatment with 0.0025 mM, or 0.005 mM of doxycycline for 24 h, followed by 24 h incubation with fresh doxycycline at the above concentrations and 0.25 μg/ml L-OspA, live, sonicated B. burgdorferi (both at 10 MOI), or not stimulant. Shown are the mean values ± SD obtained from triplicate specimens. * (P<0.05) and ** (P<0.005) indicates significant differences between untreated and doxycycline-treated cells in a dose-dependent manner. Similar results were obtained with supernatants obtained from cells purified from 3 additional rhesus macaques.
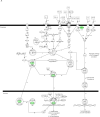
Figure 3. Microarray analysis showing the NFκB signaling pathway affected by doxycycline in stimulated THP-1 cells
Cells were incubated in medium alone or pretreated with 0.01 mM of doxycycline for 24 h, followed by incubation with fresh 0.01 mM doxycycline and sonicated B. burgdorferi (10 MOI-equivalent) for 2 or 12 h. Pathway analysis was performed using Ingenuity Pathways Analysis (IPA) software, version 5.0. Two pathways with high significance of genes regulated by doxycycline in THP-1 cells at 2 h (A) and 12 h (B) were identified and merged. Color indicates the degree of up- (red) or down-regulation (green) of the genes identified by microarray analysis and others are those associated with the regulated genes based on the pathway analysis.
Figure 3. Microarray analysis showing the NFκB signaling pathway affected by doxycycline in stimulated THP-1 cells
Cells were incubated in medium alone or pretreated with 0.01 mM of doxycycline for 24 h, followed by incubation with fresh 0.01 mM doxycycline and sonicated B. burgdorferi (10 MOI-equivalent) for 2 or 12 h. Pathway analysis was performed using Ingenuity Pathways Analysis (IPA) software, version 5.0. Two pathways with high significance of genes regulated by doxycycline in THP-1 cells at 2 h (A) and 12 h (B) were identified and merged. Color indicates the degree of up- (red) or down-regulation (green) of the genes identified by microarray analysis and others are those associated with the regulated genes based on the pathway analysis.
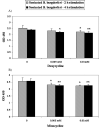
Figure 4. DNA-binding activity of NFκB in untreated and doxycycline/minocycline-treated stimulated THP-1 cells
The activity of NFκB subunit p65 in THP-1 cells was measured using the TransAM ELISA kit. Cells were incubated in medium alone or treated with doxycycline or minocycline at a concentration of 0.005 mM, or 0.01 mM, followed by incubation with sonicated B. burgdorferi (10 MOI-equivalent) for 2h or 4h. Shown are the mean values obtained from triplicate specimens ± SD. *(P<0.05) and **(P<0.005) indicate a significant difference between untreated and doxycycline/minocycline-treated cells in a dose-dependent manner.
Figure 5. Western blot analysis showing the effects of doxycycline on IκBα phosphorylation
THP-1 cells were pre-treated for 24 hours with doxycycline at 0.01 mM, washed, and then incubated with fresh doxycycline followed by stimulation with sonicated B. burgdorferi for 15, 30, or 60 min. IκBα phosphorylation was determined by Western blot (A) using anti-phospho-IκBα antibody; β-tubulin was the control for equal protein loading. Similar results were obtained in three independent experiments.
Similar articles
- Critical analysis of treatment trials of rhesus macaques infected with Borrelia burgdorferi reveals important flaws in experimental design.
Wormser GP, Baker PJ, O'Connell S, Pachner AR, Schwartz I, Shapiro ED. Wormser GP, et al. Vector Borne Zoonotic Dis. 2012 Jul;12(7):535-8. doi: 10.1089/vbz.2012.1012. Epub 2012 May 23. Vector Borne Zoonotic Dis. 2012. PMID: 22620495 Free PMC article. Review. - Interaction of the Lyme disease spirochete Borrelia burgdorferi with brain parenchyma elicits inflammatory mediators from glial cells as well as glial and neuronal apoptosis.
Ramesh G, Borda JT, Dufour J, Kaushal D, Ramamoorthy R, Lackner AA, Philipp MT. Ramesh G, et al. Am J Pathol. 2008 Nov;173(5):1415-27. doi: 10.2353/ajpath.2008.080483. Epub 2008 Oct 2. Am J Pathol. 2008. PMID: 18832582 Free PMC article. - In vitro susceptibility of Borrelia burgdorferi isolates to three antibiotics commonly used for treating equine Lyme disease.
Caol S, Divers T, Crisman M, Chang YF. Caol S, et al. BMC Vet Res. 2017 Sep 29;13(1):293. doi: 10.1186/s12917-017-1212-3. BMC Vet Res. 2017. PMID: 28962614 Free PMC article. - Effects of dexamethasone and meloxicam on Borrelia burgdorferi-induced inflammation in glial and neuronal cells of the central nervous system.
Ramesh G, Martinez AN, Martin DS, Philipp MT. Ramesh G, et al. J Neuroinflammation. 2017 Feb 2;14(1):28. doi: 10.1186/s12974-017-0806-9. J Neuroinflammation. 2017. PMID: 28153013 Free PMC article. - Therapy for Lyme arthritis: strategies for the treatment of antibiotic-refractory arthritis.
Steere AC, Angelis SM. Steere AC, et al. Arthritis Rheum. 2006 Oct;54(10):3079-86. doi: 10.1002/art.22131. Arthritis Rheum. 2006. PMID: 17009226 Review. No abstract available.
Cited by
- The Impact of Doxycycline as an Adjunctive Therapy on Prostate-Specific Antigen, Quality of Life, and Cognitive Function in Metastatic Prostate Cancer Patients: A Phase II Randomized Controlled Trial.
Guzmán-Esquivel J, Garcia-Garcia HS, Hernández-Fuentes GA, Venegas-Ramírez J, Barajas-Mejía CD, Garza-Veloz I, Martinez-Fierro ML, Magaña-Vergara NE, Guzmán-Solórzano JA, Calvo-Soto P, Avila-Zamora ON, Fuentes-Murguia M, Ceja-Espíritu G, Delgado-Enciso I. Guzmán-Esquivel J, et al. Pharmaceutics. 2025 Mar 24;17(4):404. doi: 10.3390/pharmaceutics17040404. Pharmaceutics. 2025. PMID: 40284400 Free PMC article. - Stimulated Immune Response by TruCulture® Whole Blood Assay in Patients With European Lyme Neuroborreliosis: A Prospective Cohort Study.
Ørbæk M, Gynthersen RMM, Mens H, Stenør C, Wiese L, Brandt C, Ostrowski SR, Nielsen SD, Lebech AM. Ørbæk M, et al. Front Cell Infect Microbiol. 2021 May 10;11:666037. doi: 10.3389/fcimb.2021.666037. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34041044 Free PMC article. - The conjunctival transcriptome in Ethiopians after trichiasis surgery: associations with the development of eyelid contour abnormalities and the effect of oral doxycycline treatment.
Derrick T, Habtamu E, Tadesse Z, Callahan EK, Worku A, Gashaw B, Macleod D, Mabey DCW, Holland MJ, Burton MJ. Derrick T, et al. Wellcome Open Res. 2022 Nov 11;4:130. doi: 10.12688/wellcomeopenres.15419.2. eCollection 2019. Wellcome Open Res. 2022. PMID: 37426632 Free PMC article. - Chitosan/PVA/Doxycycline Film and Nanofiber Accelerate Diabetic Wound Healing in a Rat Model.
Hedayatyanfard K, Bagheri Khoulenjani S, Abdollahifar MA, Amani D, Habibi B, Zare F, Asadirad A, Pouriran R, Ziai SA. Hedayatyanfard K, et al. Iran J Pharm Res. 2020 Fall;19(4):225-239. doi: 10.22037/ijpr.2020.112620.13859. Iran J Pharm Res. 2020. PMID: 33841538 Free PMC article. - Lyme disease and pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS): an overview.
Rhee H, Cameron DJ. Rhee H, et al. Int J Gen Med. 2012;5:163-74. doi: 10.2147/IJGM.S24212. Epub 2012 Feb 22. Int J Gen Med. 2012. PMID: 22393303 Free PMC article.
References
- Kenefick KB, Lim LC, Alder JD, Schmitz JL, Czuprynski CJ, Schell RF. Induction of interleukin-1 release by high- and low-passage isolates of Borrelia burgdorferi. J Infect Dis. 1993;167:1086–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P51 RR000164/RR/NCRR NIH HHS/United States
- R01 NS048952/NS/NINDS NIH HHS/United States
- U01 CI000153/CI/NCPDCID CDC HHS/United States
- RR00164/RR/NCRR NIH HHS/United States
- UO1-CI000153/CI/NCPDCID CDC HHS/United States
- NS048952/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous