HLA-G homodimer-induced cytokine secretion through HLA-G receptors on human decidual macrophages and natural killer cells - PubMed (original) (raw)
HLA-G homodimer-induced cytokine secretion through HLA-G receptors on human decidual macrophages and natural killer cells
Changlin Li et al. Proc Natl Acad Sci U S A. 2009.
Abstract
Human decidual CD14(+) macrophages and CD56(+) NK cells were isolated from material obtained after first-trimester pregnancy terminations. Each cell type expressed a specific surface receptor for histocompatibility leukocyte antigen (HLA)-G (an MHC class Ib protein that is expressed on extravillous trophoblasts), LILRB1 on CD14(+) macrophages and KIR2DL4 on CD56(+) NK cells. Cross-linking with anti-LILRB1 or anti-KIR2DL4 resulted in up-regulation of a small subset of mRNAs including those for IL-6, IL-8, and TNFalpha detected using a microarray representing 114 cytokines. Incubation with transfectants expressing the HLA-G homodimer (but not with transfectants expressing the HLA-G monomer) resulted in secretion of the same cytokine proteins from both leukocyte sets. Moreover, cytokine secretion from both leukocyte sets was blocked by both the appropriate anti-receptor mAb and by anti-HLA-G. The amount of these cytokines secreted by decidual macrophages was substantially greater than that secreted by decidual NK cells. VEGF was constitutively secreted by both cell types. LILRB1, which contains an immunoreceptor tyrosine-based switch motif, functions here as an activating receptor, although it has been known as an inhibitory receptor. KIR2DL4 also functions as an activating receptor, although it also has the potential to function as an inhibitory receptor. Secretion of proinflammatory and proangiogenic proteins supports a role for these leukocytes in important processes that are essential for successful pregnancy, but they may represent only a portion of the proteins that are secreted.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
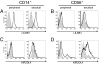
Fig. 1.
LILRB1 and KIR2DL4 surface expression on peripheral and decidual CD14+ and CD56+ cells assessed by flow cytometry. (A) LILRB1 expression on peripheral and decidual CD14+ cells. (B) LILRB1 expression on peripheral and decidual CD56+ cells. (C) KIR2DL4 expression on peripheral and decidual CD14+ cells. (D) KIR2DL4 expression on peripheral and decidual CD56+ cells. Staining relative to an isotype control antibody (shaded) is shown.
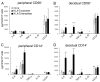
Fig. 2.
HLA-G homodimer induced cytokine protein secretion by human peripheral and decidual CD14+ macrophages and CD56+ NK cells. Peripheral CD56+ (A), decidual CD56+ cells (B), peripheral CD14+ cells (C), or decidual CD14+ cells (D) were cocultured in a 1:1 ratio with HLA class I-negative 721.221 cells (white bars), 721.221 cells transfected to express HLA-G monomer (gray bars), or 721.221cells transfected to express HLA-G homodimer (black bars). Supernatants were collected after 24 h for CD14+ macrophages or after 48 h for CD56+ NK cells. The concentration of cytokines (IL-6, IL-8, IL-10, TNFα, or VEGF) in each supernatant was measured by a multiplex cytokine assay. The SD was calculated from the mean of 4 experiments, each with an individual donor. Statistical significance: *, P < 0.05; **, P < 0.01; and ***, P < 0.001. Note that the scale of C is compressed ≈4-fold relative to D because the secretion of IL-8 by peripheral CD14+ cells is so much larger than that of decidual CD14+ cells.
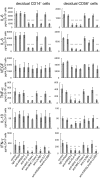
Fig. 3.
Cytokine secretion induced by the HLA-G homodimer was blocked in the presence of specific antibodies against HLA-G or its receptors. Decidual CD14+ cells (Left) or decidual CD56+ cells (Right) were cocultured in a 1:1 ratio with 721.221 cells transfected with HLA-G homodimer in the presence of various mAb as shown. Supernatants were collected after 24 h for CD14+ macrophages or after 48 h for CD56+ NK cells. The concentration of cytokines (IL-6, IL-8, IL-10, IFNγ, TNFα, or VEGF) in each supernatant was measured by a multiplex cytokine assay. The SD was calculated from the mean of 3 experiments for decidual CD56+ cells and 4 experiments for decidual CD14+ cells, each with an individual donor. Statistical significance: *, P < 0.05; **, P < 0.01; and ***, P < 0.001.
Similar articles
- A human histocompatibility leukocyte antigen (HLA)-G-specific receptor expressed on all natural killer cells.
Rajagopalan S, Long EO. Rajagopalan S, et al. J Exp Med. 1999 Apr 5;189(7):1093-100. doi: 10.1084/jem.189.7.1093. J Exp Med. 1999. PMID: 10190900 Free PMC article. - Triiodothyronine regulates angiogenic growth factor and cytokine secretion by isolated human decidual cells in a cell-type specific and gestational age-dependent manner.
Vasilopoulou E, Loubière LS, Lash GE, Ohizua O, McCabe CJ, Franklyn JA, Kilby MD, Chan SY. Vasilopoulou E, et al. Hum Reprod. 2014 Jun;29(6):1161-72. doi: 10.1093/humrep/deu046. Epub 2014 Mar 13. Hum Reprod. 2014. PMID: 24626803 Free PMC article. - Human KIR2DL5 is an inhibitory receptor expressed on the surface of NK and T lymphocyte subsets.
Estefanía E, Flores R, Gómez-Lozano N, Aguilar H, López-Botet M, Vilches C. Estefanía E, et al. J Immunol. 2007 Apr 1;178(7):4402-10. doi: 10.4049/jimmunol.178.7.4402. J Immunol. 2007. PMID: 17371997 - Immunological relationship between the mother and the fetus.
Szekeres-Bartho J. Szekeres-Bartho J. Int Rev Immunol. 2002 Nov-Dec;21(6):471-95. doi: 10.1080/08830180215017. Int Rev Immunol. 2002. PMID: 12650238 Review. - Recognition of trophoblast HLA class I molecules by decidual NK cell receptors--a review.
King A, Hiby SE, Gardner L, Joseph S, Bowen JM, Verma S, Burrows TD, Loke YW. King A, et al. Placenta. 2000 Mar-Apr;21 Suppl A:S81-5. doi: 10.1053/plac.1999.0520. Placenta. 2000. PMID: 10831129 Review.
Cited by
- Cellular senescence induced by CD158d reprograms natural killer cells to promote vascular remodeling.
Rajagopalan S, Long EO. Rajagopalan S, et al. Proc Natl Acad Sci U S A. 2012 Dec 11;109(50):20596-601. doi: 10.1073/pnas.1208248109. Epub 2012 Nov 26. Proc Natl Acad Sci U S A. 2012. PMID: 23184984 Free PMC article. - Cytokines and Soluble HLA-G Levels in the Acute and Recovery Phases of Arbovirus-Infected Brazilian Patients Exhibiting Neurological Complications.
Almeida RS, Ferreira MLB, Sonon P, Cordeiro MT, Sadissou I, Diniz GTN, Militão-Albuquerque MFP, Franca RFO, Donadi EA, Lucena-Silva N. Almeida RS, et al. Front Immunol. 2021 Mar 12;12:582935. doi: 10.3389/fimmu.2021.582935. eCollection 2021. Front Immunol. 2021. PMID: 33776990 Free PMC article. - Pregnancy-Induced Alterations in NK Cell Phenotype and Function.
Le Gars M, Seiler C, Kay AW, Bayless NL, Starosvetsky E, Moore L, Shen-Orr SS, Aziz N, Khatri P, Dekker CL, Swan GE, Davis MM, Holmes S, Blish CA. Le Gars M, et al. Front Immunol. 2019 Oct 23;10:2469. doi: 10.3389/fimmu.2019.02469. eCollection 2019. Front Immunol. 2019. PMID: 31708922 Free PMC article. - DNA-PKcs controls an endosomal signaling pathway for a proinflammatory response by natural killer cells.
Rajagopalan S, Moyle MW, Joosten I, Long EO. Rajagopalan S, et al. Sci Signal. 2010 Feb 23;3(110):ra14. doi: 10.1126/scisignal.2000467. Sci Signal. 2010. PMID: 20179272 Free PMC article. - Relative expression of receptors in uterine natural killer cells compared to peripheral blood natural killer cells.
Ismail NI. Ismail NI. Front Immunol. 2023 Mar 24;14:1166451. doi: 10.3389/fimmu.2023.1166451. eCollection 2023. Front Immunol. 2023. PMID: 37051244 Free PMC article. Review.
References
- Medawar PB. Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates. Symp Soc Exp Biol. 1953;7:320–338.
- Faulk WP, Temple A. Distribution of β2-microglobulin and HLA in chorionic villi of human placentae. Nature. 1976;262:799–802. - PubMed
- Orr HT, et al. Use of HLA loss mutants to analyze the structure of the human major histocompatibility complex. Nature. 1982;296:454–456. - PubMed
- King A, et al. HLA-E is expressed on trophoblast and interacts with CD94/NKG2 receptors on decidual NK cells. Eur J Immunol. 2000;30:1623–1631. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials