Hepatic triacylglycerol hydrolysis regulates peroxisome proliferator-activated receptor alpha activity - PubMed (original) (raw)
Hepatic triacylglycerol hydrolysis regulates peroxisome proliferator-activated receptor alpha activity
Jessica M Sapiro et al. J Lipid Res. 2009 Aug.
Abstract
Recent evidence suggests that fatty acids generated from intracellular triacylglycerol (TAG) hydrolysis may have important roles in intracellular signaling. This study was conducted to determine if fatty acids liberated from TAG hydrolysis regulate peroxisome proliferator-activated receptor alpha (PPARalpha). Primary rat hepatocyte cultures were treated with adenoviruses overexpressing adipose differentiation-related protein (ADRP) or adipose triacylglycerol lipase (ATGL) or treated with short interfering RNA (siRNA) targeted against ADRP. Subsequent effects on TAG metabolism and PPARalpha activity and target gene expression were determined. Overexpressing ADRP attenuated TAG hydrolysis, whereas siRNA-mediated knockdown of ADRP or ATGL overexpression resulted in enhanced TAG hydrolysis. Results from PPARalpha reporter activity assays demonstrated that decreasing TAG hydrolysis by ADRP overexpression resulted in a 35-60% reduction in reporter activity under basal conditions or in the presence of fatty acids. As expected, PPARalpha target genes were also decreased in response to ADRP overexpression. However, the PPARalpha ligand, WY-14643, was able to restore PPARalpha activity following ADRP overexpression. Despite its effects on PPARalpha, overexpressing ADRP did not affect PPARgamma activity. Enhancing TAG hydrolysis through ADRP knockdown or ATGL overexpression increased PPARalpha activity. These results indicate that TAG hydrolysis and the consequential release of fatty acids regulate PPARalpha activity.
Figures
Fig. 1.
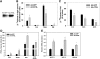
ADRP overexpression decreases the loss of fatty acids from TAG and inhibits PPARα activity and target gene expression. A: Western blot analysis of ADRP overexpression showing endogenous rat ADRP and overexpressed murine FLAG-tagged ADRP, which ran approximately 2 kDa higher during electrophoresis. B: Incorporation of [1-14C]oleate into cellular lipids. C–E: Chase experiments evaluating turnover of TAG, phospholipids, and cholesterol esters. Statistics for the chase period were analyzed as a percentage of the pulse. F: PPARα reporter gene activity following exposure to fatty acids. G: Expression of PPARα target genes. All data are reported as means ± SE from three to four experiments. *P < 0.05 and **P < 0.01 when compared with Ad-GFP controls.
Fig. 2.
ADRP knockdown enhances fatty acid loss from intracellular TAG and increases PPARα activity. A: ADRP protein abundance measured with Western blotting at 48 h after transfection. B: Eight hours after siRNA transfection, cells were lysed and RNA was harvested and analyzed for ADRP mRNA abundance with qRT-PCR. C: Incorporation of [1-14C]oleate into cellular lipids. D: Chase experiments evaluating turnover of lipid species. Statistics for the chase period were analyzed as a percentage of the pulse. Statistics for the chase period were analyzed as a percentage of the pulse. E: PPARα reporter gene activity following exposure to fatty acids. Data are reported as means ± SE, n = 3. *P < 0.05, **P < 0.01, and ***P < 0.001 when compared with siRNA controls.
Fig. 3.
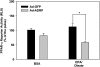
Overexpressing ADRP decreases PPARα reporter activity following removal of exogenous fatty acids. Fourteen hours after transduction, cells were exposed to a combination of 250 μM EPA and 250 μM oleate for 6 h (pulse). The media was removed and replaced with media devoid of fatty acids for an additional 6 h (chase). Reporter gene assays were performed immediately after removal of exogenous fatty acids and 6 h later. Data are presented as PPARα reporter activity after the 6 h chase period expressed as a percentage of the pulse period. Data are reported as means ± SE, n = 3. *P < 0.05 when compared with Ad-GFP controls.
Fig. 4.
Overexpressing ATGL enhances the loss of fatty acids from TAG and increases PPARα activity and target gene expression. A: Western blot analysis of ATGL overexpression. B: Incorporation of [1-14C]oleate into cellular lipids. C: Chase experiments evaluating turnover of TAG. Statistics for the chase period were analyzed as a percentage of the pulse. D: PPARα reporter gene activity following exposure to fatty acids. E: Expression of PPARα target genes. All data are reported as means ± SE, n = 3. *P < 0.05 and **P < 0.01 when compared with Ad-GFP controls.
Fig. 5.
The PPARα ligand, WY-14643, normalizes PPARα activity in cells overexpressing ADRP. At 24 h after viral transduction, cells were treated with DMSO or 1 μM WY-14643 for 6 h prior to harvesting for reporter gene assays. Data are reported as means ± SE, n = 3. *P < 0.05 when compared with Ad-GFP controls.
Fig. 6.
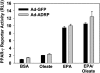
ADRP overexpression does not alter PPARγ activity. PPARγ reporter gene activity following exposure to fatty acids. Data are reported as means ± SE, n = 3. There were no significant differences between Ad-GFP and Ad-ADRP transduced cells.
Similar articles
- PPARalpha activation increases triglyceride mass and adipose differentiation-related protein in hepatocytes.
Edvardsson U, Ljungberg A, Lindén D, William-Olsson L, Peilot-Sjögren H, Ahnmark A, Oscarsson J. Edvardsson U, et al. J Lipid Res. 2006 Feb;47(2):329-40. doi: 10.1194/jlr.M500203-JLR200. Epub 2005 Nov 10. J Lipid Res. 2006. PMID: 16282640 - Adipose triglyceride lipase is a major hepatic lipase that regulates triacylglycerol turnover and fatty acid signaling and partitioning.
Ong KT, Mashek MT, Bu SY, Greenberg AS, Mashek DG. Ong KT, et al. Hepatology. 2011 Jan;53(1):116-26. doi: 10.1002/hep.24006. Epub 2010 Oct 21. Hepatology. 2011. PMID: 20967758 Free PMC article. - VLDL hydrolysis by hepatic lipase regulates PPARδ transcriptional responses.
Brown JD, Oligino E, Rader DJ, Saghatelian A, Plutzky J. Brown JD, et al. PLoS One. 2011;6(7):e21209. doi: 10.1371/journal.pone.0021209. Epub 2011 Jul 5. PLoS One. 2011. PMID: 21750705 Free PMC article. - Peroxisome proliferator-activated receptor delta regulates lipid droplet formation and transport in goat mammary epithelial cells.
Shi HB, Zhang CH, Xu ZA, Lou GG, Liu JX, Luo J, Loor JJ. Shi HB, et al. J Dairy Sci. 2018 Mar;101(3):2641-2649. doi: 10.3168/jds.2017-13543. Epub 2018 Jan 10. J Dairy Sci. 2018. PMID: 29331469 - Fatty acid synthase and liver triglyceride metabolism: housekeeper or messenger?
Jensen-Urstad AP, Semenkovich CF. Jensen-Urstad AP, et al. Biochim Biophys Acta. 2012 May;1821(5):747-53. doi: 10.1016/j.bbalip.2011.09.017. Epub 2011 Oct 8. Biochim Biophys Acta. 2012. PMID: 22009142 Free PMC article. Review.
Cited by
- Impact of Reduced ATGL-Mediated Adipocyte Lipolysis on Obesity-Associated Insulin Resistance and Inflammation in Male Mice.
Schoiswohl G, Stefanovic-Racic M, Menke MN, Wills RC, Surlow BA, Basantani MK, Sitnick MT, Cai L, Yazbeck CF, Stolz DB, Pulinilkunnil T, O'Doherty RM, Kershaw EE. Schoiswohl G, et al. Endocrinology. 2015 Oct;156(10):3610-24. doi: 10.1210/en.2015-1322. Epub 2015 Jul 21. Endocrinology. 2015. PMID: 26196542 Free PMC article. - Perilipin 5: putting the brakes on lipolysis.
Brasaemle DL. Brasaemle DL. J Lipid Res. 2013 Apr;54(4):876-7. doi: 10.1194/jlr.E036962. Epub 2013 Feb 17. J Lipid Res. 2013. PMID: 23417737 Free PMC article. No abstract available. - Suppression of long chain acyl-CoA synthetase 3 decreases hepatic de novo fatty acid synthesis through decreased transcriptional activity.
Bu SY, Mashek MT, Mashek DG. Bu SY, et al. J Biol Chem. 2009 Oct 30;284(44):30474-83. doi: 10.1074/jbc.M109.036665. Epub 2009 Sep 8. J Biol Chem. 2009. PMID: 19737935 Free PMC article. - ATGL Promotes Autophagy/Lipophagy via SIRT1 to Control Hepatic Lipid Droplet Catabolism.
Sathyanarayan A, Mashek MT, Mashek DG. Sathyanarayan A, et al. Cell Rep. 2017 Apr 4;19(1):1-9. doi: 10.1016/j.celrep.2017.03.026. Cell Rep. 2017. PMID: 28380348 Free PMC article. - Cyclin D1 represses peroxisome proliferator-activated receptor alpha and inhibits fatty acid oxidation.
Kamarajugadda S, Becker JR, Hanse EA, Mashek DG, Mashek MT, Hendrickson AM, Mullany LK, Albrecht JH. Kamarajugadda S, et al. Oncotarget. 2016 Jul 26;7(30):47674-47686. doi: 10.18632/oncotarget.10274. Oncotarget. 2016. PMID: 27351284 Free PMC article.
References
- Ducharme N. A., Bickel P. E. 2008. Minireview: lipid droplets in lipogenesis and lipolysis. Endocrinology. 149: 942–949 - PubMed
- Jump D. B., Botolin D., Wang Y., Xu J., Christian B., Demeure O. 2005. Fatty acid regulation of hepatic gene transcription. J. Nutr. 135: 2503–2506 - PubMed
- Beaven S. W., Tontonoz P. 2006. Nuclear receptors in lipid metabolism: targeting the heart of dyslipidemia. Annu. Rev. Med. 57: 313–329 - PubMed
- Pawar A., Jump D. B. 2003. Unsaturated fatty acid regulation of peroxisome proliferator-activated receptor alpha activity in rat primary hepatocytes. J. Biol. Chem. 278: 35931–35939 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials