Heparanase regulates levels of syndecan-1 in the nucleus - PubMed (original) (raw)
Heparanase regulates levels of syndecan-1 in the nucleus
Ligong Chen et al. PLoS One. 2009.
Abstract
Syndecan-1 is a transmembrane heparan sulfate-bearing proteoglycan known to regulate multiple biological functions at the cell surface and within the extracellular matrix. Its functional activity can be modulated by heparanase, an enzyme that cleaves heparan sulfate chains and whose expression has been associated with an aggressive phenotype in many cancers. In addition to remodeling syndecan-1 by cleaving its heparan sulfate chains, heparanase influences syndecan-1 location by upregulating expression of enzymes that accelerate its shedding from the cell surface. In the present study we discovered that heparanase also alters the level of nuclear syndecan-1. Upon upregulation of heparanase expression or following addition of recombinant heparanase to myeloma cells, the nuclear localization of syndecan-1 drops dramatically as revealed by confocal microscopy, western blotting and quantification by ELISA. This effect requires enzymatically active heparanase because cells expressing high levels of mutated, enzymatically inactive heparanase, failed to diminish syndecan-1 levels in the nucleus. Although heparan sulfate function within the nucleus is not well understood, there is emerging evidence that it may act to repress transcriptional activity. The resulting changes in gene expression facilitated by the loss of nuclear syndecan-1 could explain how heparanase enhances expression of MMP-9, VEGF, tissue factor and perhaps other effectors that condition the tumor microenvironment to promote an aggressive cancer phenotype.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
Figure 1. Syndecan-1 is not detected within the nucleus of cells expressing high levels of heparanase.
Confocal microscopic z-stack images of (A) HPSE-low and (B) HPSE-high cells immunostained using antibody to syndecan-1. Blue (Hoechst stain) identifies nuclei; white identifies syndecan-1 within the nucleus (co-localization of Hoechst and syndecan-1); green identifies cytoplasmic and cell surface syndecan-1. Syndecan-1 is detected within nuclei of HPSE-low cells but absent in nuclei of the HPSE-high cells. Bar = 10 µm. Note: As is characteristic of myeloma cells, the size of the nucleus is large relative to the amount of cytoplasm.
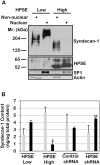
Figure 2. Elevated expression of heparanase dramatically decreases the level of syndecan-1 present within the nucleus.
A) Nuclear and non-nuclear fractions were isolated from HPSE-low and HPSE-high cells (prepared using the pcDNA3 vector for transfections) and separated on SDS-PAGE. Western blots were probed with antibody to human syndecan-1, human heparanase, SP-1 or actin. B) Nuclear and non-nuclear fractions were isolated from HPSE-low and HPSE-high cells (prepared using the pIRES2 vector for transfections) and from wild-type CAG cells infected with control shRNA or an shRNA targeting heparanase. The quantity of syndecan-1 in each fraction was determined by ELISA. Grey bars = non-nuclear fraction; Black bars = nuclear fraction. Error bars represent standard error of the mean. *, P<0.01 vs. nuclear syndecan-1 in HPSE low cells; **, P<0.02 vs. nuclear syndecan-1 in shRNA control.
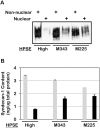
Figure 3. Heparanase enzymatic activity is required for reduction of syndecan-1 levels in the nucleus.
Nuclear and non-nuclear fractions were prepared from CAG cells expressing high levels of wild-type heparanase (HPSE-high) or heparanase mutated at either amino acid 343 (M343) or amino acid 225 (M225) which renders them enzymatically inactive. All cells were prepared using pIRES2 vectors for transfections. Fractions were analyzed for syndecan-1 levels by A) western blotting and B) ELISA. Grey bars = non-nuclear fraction; Black bars = nuclear fraction. Error bars represent standard error of the mean. *, P<0.01 vs. nuclear syndecan-1 in HPSE high cells.
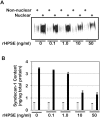
Figure 4. Exogenous recombinant heparanase (rHPSE) decreases nuclear syndecan-1 levels in a concentration-dependent manner.
Recombinant heparanase was added to CAG cells having very low levels of heparanase expression (shRNA knockdown cells). Nuclear and non-nuclear fractions were prepared and syndecan-1 levels analyzed by A) western blotting and B) ELISA. Grey bars = non-nuclear fraction; Black bars = nuclear fraction. Error bars represent standard error of the mean. *, P<0.01 vs. nuclear syndecan-1 in cells treated with 0 ng/ml rHPSE.
Similar articles
- Heparanase-mediated loss of nuclear syndecan-1 enhances histone acetyltransferase (HAT) activity to promote expression of genes that drive an aggressive tumor phenotype.
Purushothaman A, Hurst DR, Pisano C, Mizumoto S, Sugahara K, Sanderson RD. Purushothaman A, et al. J Biol Chem. 2011 Sep 2;286(35):30377-30383. doi: 10.1074/jbc.M111.254789. Epub 2011 Jul 11. J Biol Chem. 2011. PMID: 21757697 Free PMC article. - Heparanase enhances syndecan-1 shedding: a novel mechanism for stimulation of tumor growth and metastasis.
Yang Y, Macleod V, Miao HQ, Theus A, Zhan F, Shaughnessy JD Jr, Sawyer J, Li JP, Zcharia E, Vlodavsky I, Sanderson RD. Yang Y, et al. J Biol Chem. 2007 May 4;282(18):13326-33. doi: 10.1074/jbc.M611259200. Epub 2007 Mar 8. J Biol Chem. 2007. PMID: 17347152 - Heparanase-1-induced shedding of heparan sulfate from syndecan-1 in hepatocarcinoma cell facilitates lymphatic endothelial cell proliferation via VEGF-C/ERK pathway.
Yu S, Lv H, Zhang H, Jiang Y, Hong Y, Xia R, Zhang Q, Ju W, Jiang L, Ou G, Zhang J, Wang S, Zhang J. Yu S, et al. Biochem Biophys Res Commun. 2017 Apr 1;485(2):432-439. doi: 10.1016/j.bbrc.2017.02.060. Epub 2017 Feb 13. Biochem Biophys Res Commun. 2017. PMID: 28209511 - The heparanase/syndecan-1 axis in cancer: mechanisms and therapies.
Ramani VC, Purushothaman A, Stewart MD, Thompson CA, Vlodavsky I, Au JL, Sanderson RD. Ramani VC, et al. FEBS J. 2013 May;280(10):2294-306. doi: 10.1111/febs.12168. Epub 2013 Mar 4. FEBS J. 2013. PMID: 23374281 Free PMC article. Review. - Heparanase-enhanced Shedding of Syndecan-1 and Its Role in Driving Disease Pathogenesis and Progression.
Rangarajan S, Richter JR, Richter RP, Bandari SK, Tripathi K, Vlodavsky I, Sanderson RD. Rangarajan S, et al. J Histochem Cytochem. 2020 Dec;68(12):823-840. doi: 10.1369/0022155420937087. Epub 2020 Jul 6. J Histochem Cytochem. 2020. PMID: 32623935 Free PMC article. Review.
Cited by
- New classes of potent heparanase inhibitors from ligand-based virtual screening.
Pala D, Scalvini L, Elisi GM, Lodola A, Mor M, Spadoni G, Ferrara FF, Pavoni E, Roscilli G, Milazzo FM, Battistuzzi G, Rivara S, Giannini G. Pala D, et al. J Enzyme Inhib Med Chem. 2020 Dec;35(1):1685-1696. doi: 10.1080/14756366.2020.1811701. J Enzyme Inhib Med Chem. 2020. PMID: 32907434 Free PMC article. - Heparanase enhances the insulin receptor signaling pathway to activate extracellular signal-regulated kinase in multiple myeloma.
Purushothaman A, Babitz SK, Sanderson RD. Purushothaman A, et al. J Biol Chem. 2012 Nov 30;287(49):41288-96. doi: 10.1074/jbc.M112.391417. Epub 2012 Oct 9. J Biol Chem. 2012. PMID: 23048032 Free PMC article. - Dually modified transmembrane proteoglycans in development and disease.
Jenkins LM, Horst B, Lancaster CL, Mythreye K. Jenkins LM, et al. Cytokine Growth Factor Rev. 2018 Feb;39:124-136. doi: 10.1016/j.cytogfr.2017.12.003. Epub 2017 Dec 22. Cytokine Growth Factor Rev. 2018. PMID: 29291930 Free PMC article. Review. - The niche factor syndecan-1 regulates the maintenance and proliferation of neural progenitor cells during mammalian cortical development.
Wang Q, Yang L, Alexander C, Temple S. Wang Q, et al. PLoS One. 2012;7(8):e42883. doi: 10.1371/journal.pone.0042883. Epub 2012 Aug 24. PLoS One. 2012. PMID: 22936997 Free PMC article. - The Unifying Hypothesis of Alzheimer's Disease: Heparan Sulfate Proteoglycans/Glycosaminoglycans Are Key as First Hypothesized Over 30 Years Ago.
Snow AD, Cummings JA, Lake T. Snow AD, et al. Front Aging Neurosci. 2021 Oct 4;13:710683. doi: 10.3389/fnagi.2021.710683. eCollection 2021. Front Aging Neurosci. 2021. PMID: 34671250 Free PMC article.
References
- Ilan N, Elkin M, Vlodavsky I. Regulation, function and clinical significance of heparanase in cancer metastasis and angiogenesis. Int J Biochem Cell Biol. 2006;38:2018–2039. - PubMed
- Vlodavsky I, Elkin M, Abboud-Jarrous G, Levi-Adam F, Fuks L, et al. Heparanase: one molecule with multiple functions in cancer progression. Connect Tissue Res. 2008;49:207–210. - PubMed
- Yang Y, Macleod V, Miao HQ, Theus A, Zhan F, et al. Heparanase enhances syndecan-1 shedding: A novel mechanism for stimulation of tumor growth and metastasis. J Biol Chem. 2007;282:13326–13333. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA013148/CA/NCI NIH HHS/United States
- P30 CA013148/CA/NCI NIH HHS/United States
- R01 CA135075/CA/NCI NIH HHS/United States
- R01 CA103054/CA/NCI NIH HHS/United States
- CA103054/CA/NCI NIH HHS/United States
- CA135075/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous