Connexin43 phosphorylation: structural changes and biological effects - PubMed (original) (raw)
Review
Connexin43 phosphorylation: structural changes and biological effects
Joell L Solan et al. Biochem J. 2009.
Abstract
Vertebrate gap junctions, composed of proteins from the connexin gene family, play critical roles in embryonic development, co-ordinated contraction of excitable cells, tissue homoeostasis, normal cell growth and differentiation. Phosphorylation of connexin43, the most abundant and ubiquitously expressed connexin, has been implicated in the regulation of gap junctional communication at several stages of the connexin 'life cycle', including hemichannel oligomerization, export of the protein to the plasma membrane, hemichannel activity, gap junction assembly, gap junction channel gating and connexin degradation. Consistent with a short (1-5 h) protein half-life, connexin43 phosphorylation is dynamic and changes in response to activation of many different kinases. The present review assesses our current understanding of the effects of phosphorylation on connexin43 structure and function that in turn regulate gap junction biology, with an emphasis on events occurring in heart and skin.
Figures
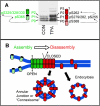
Figure 1. Phosphorylation and assembly of Cx43 containing gap junctions
(A) Association of the SDS-PAGE migration of Cx43 in homeostatic (CON) and TPA treated cells with the sites of phosphorylation and a schematic diagram representing channel activity. The cylindrical channels are green to represent promoting communication and red to represent inhibition or closure. The green cross hatch represents an assembling channel and the red a channel with reduced conductance. (B) Gap junction assembly is denoted by cross hatch green channels assembling into solid green communicating channels and red channels represent those that will be internalized and degraded.
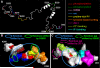
Figure 2. Model of the Cx43 C-terminus based on NMR structural studies
(A) Cartoon of a possible structure for the Cx43 C-terminus (amino acids 252−382) with known regulatory sites/binding sequences marked in different colors produced using the PyMOL Molecular Graphics System (
). (B and C) Space filled models of amino acids 260−292 and 360−382, respectively. Circles mark residues which show resonance peak shifts in NMR spectra in response to SH3 binding (B) or S365D substitution (C). Blue circles mark residues that shift in response binding of the src SH3 domain (B and C). Green circles mark residues that are shifted in a S365D mutant as compared to wild type (B). In (C) dashed lines represent the only residues shown that do not shift due to ZO-1 binding (pink) or the S365D mutation (yellow).
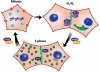
Figure 3. Connexin localization and phosphorylation change dramatically during the cell cycle
Confluent cells in G0/G1 are very efficient at trafficking and assembling (green arrow) most of the Cx43 into Triton X-100 insoluble gap junctions, shown in green. ZO-1 and ZO-2 are colocalized at these plaques. As cells progress into S phase assembly of gap junctions becomes less efficient and Cx43 is found both in gap junction plaques and in cytoplasmic vesicles (green). Association with ZO-1 is decreased while ZO-2 interaction is maintained. PKC-mediated phosphorylation of Cx43 on S368 and S262 (transient?) begins occurring as cells approach S phase. As cells enter mitosis, gap junction communication ceases (red) and Cx43 is found predominantly in clusters of vesicles in the cytoplasm (green). Cx43 becomes increasingly phosphorylated on S368 and S255 and exhibits a migration shift by SDS-PAGE. Cells are able to quickly resume communication upon cytokinesis, likely due to plasma membrane connexon pools.
Figure 4. Schematic diagram of connexin expression in normal and wounded human skin
Cx43 expression (denoted in green) in unwounded skin is mainly in the upper more differentiated layers. Upon wounding Cx43 expression drops very near the wound and redistributes to lower layers in the cells several cells distant from the wound. The basal cells near the wound express Cx43 that is highly phosphorylated at S368 (indicated by orange) forming a distinct communication compartment.
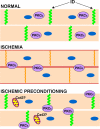
Figure 5. Schematic diagram of changes in connexin expression in heart under ischemic conditions alone or with preconditioning
Normally cardiac myocytes express high levels of Cx43 at the intercalated disc (ID) and gap junctions are in their open state, shown in green. Under ischemic conditions there is a loss of gap junctions at the intercalated disc as Cx43 moves to the lateral edges of the myocyte, shown in red. Some Cx43 is retained at the intercalated disc, but it becomes phosphorylated on S368 (indicated by orange). In addition, PKCε translocates from the cytoplasm to the plasma membrane and may be involved in Cx43 phosphorylation on S368. With ischemic preconditioning, gap junctions are retained at the intercalated disc with no subsequent S368 phosphorylation. PKCε is necessary for this retention and is found at the plasma membrane. In addition, increased Cx43 may be found in the mitochondria.
Similar articles
- Connexin phosphorylation as a regulatory event linked to gap junction channel assembly.
Solan JL, Lampe PD. Solan JL, et al. Biochim Biophys Acta. 2005 Jun 10;1711(2):154-63. doi: 10.1016/j.bbamem.2004.09.013. Epub 2004 Oct 12. Biochim Biophys Acta. 2005. PMID: 15955300 Review. - Connexin43 phosphorylation in brain, cardiac, endothelial and epithelial tissues.
Márquez-Rosado L, Solan JL, Dunn CA, Norris RP, Lampe PD. Márquez-Rosado L, et al. Biochim Biophys Acta. 2012 Aug;1818(8):1985-92. doi: 10.1016/j.bbamem.2011.07.028. Epub 2011 Jul 26. Biochim Biophys Acta. 2012. PMID: 21819962 Free PMC article. Review. - Kinase programs spatiotemporally regulate gap junction assembly and disassembly: Effects on wound repair.
Solan JL, Lampe PD. Solan JL, et al. Semin Cell Dev Biol. 2016 Feb;50:40-8. doi: 10.1016/j.semcdb.2015.12.010. Epub 2015 Dec 17. Semin Cell Dev Biol. 2016. PMID: 26706150 Free PMC article. Review. - Regulation of gap junctions by tyrosine protein kinases.
Warn-Cramer BJ, Lau AF. Warn-Cramer BJ, et al. Biochim Biophys Acta. 2004 Mar 23;1662(1-2):81-95. doi: 10.1016/j.bbamem.2003.10.018. Biochim Biophys Acta. 2004. PMID: 15033580 Free PMC article. Review. - Functional analysis of amino acid sequences in connexin43 involved in intercellular communication through gap junctions.
Becker DL, Evans WH, Green CR, Warner A. Becker DL, et al. J Cell Sci. 1995 Apr;108 ( Pt 4):1455-67. doi: 10.1242/jcs.108.4.1455. J Cell Sci. 1995. PMID: 7615666
Cited by
- Connexin- and pannexin-based channels in normal skeletal muscles and their possible role in muscle atrophy.
Cea LA, Riquelme MA, Cisterna BA, Puebla C, Vega JL, Rovegno M, Sáez JC. Cea LA, et al. J Membr Biol. 2012 Aug;245(8):423-36. doi: 10.1007/s00232-012-9485-8. Epub 2012 Aug 1. J Membr Biol. 2012. PMID: 22850938 Review. - The Multifaceted Role of Astrocyte Connexin 43 in Ischemic Stroke Through Forming Hemichannels and Gap Junctions.
Liang Z, Wang X, Hao Y, Qiu L, Lou Y, Zhang Y, Ma D, Feng J. Liang Z, et al. Front Neurol. 2020 Jul 31;11:703. doi: 10.3389/fneur.2020.00703. eCollection 2020. Front Neurol. 2020. PMID: 32849190 Free PMC article. Review. - Absence of venous valves in mice lacking Connexin37.
Munger SJ, Kanady JD, Simon AM. Munger SJ, et al. Dev Biol. 2013 Jan 15;373(2):338-48. doi: 10.1016/j.ydbio.2012.10.032. Epub 2012 Nov 7. Dev Biol. 2013. PMID: 23142761 Free PMC article. - A myocardial infarct border-zone-on-a-chip demonstrates distinct regulation of cardiac tissue function by an oxygen gradient.
Rexius-Hall ML, Khalil NN, Escopete SS, Li X, Hu J, Yuan H, Parker SJ, McCain ML. Rexius-Hall ML, et al. Sci Adv. 2022 Dec 9;8(49):eabn7097. doi: 10.1126/sciadv.abn7097. Epub 2022 Dec 7. Sci Adv. 2022. PMID: 36475790 Free PMC article. - Enhanced connexin 43 expression delays intra-mitotic duration and cell cycle traverse independently of gap junction channel function.
Johnstone SR, Best AK, Wright CS, Isakson BE, Errington RJ, Martin PE. Johnstone SR, et al. J Cell Biochem. 2010 Jun 1;110(3):772-82. doi: 10.1002/jcb.22590. J Cell Biochem. 2010. PMID: 20512937 Free PMC article.
References
- White T, Paul D. Genetic diseases and gene knockouts reveal diverse connexin functions. Ann. Rev. Physiology. 1999;61:283–310. - PubMed
- Sohl G, Willecke K. Gap junctions and the connexin protein family. Cardiovasc Res. 2004;62:228–232. - PubMed
- Saez JC, Berthoud VM, Branes MC, Martinez AD, Beyer EC. Plasma membrane channels formed by connexins: their regulation and functions. Physiol. Rev. 2003;83:1359–1400. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GM55632/GM/NIGMS NIH HHS/United States
- R01 GM055632-11/GM/NIGMS NIH HHS/United States
- R01 GM055632/GM/NIGMS NIH HHS/United States
- R01 GM055632-09/GM/NIGMS NIH HHS/United States
- R01 GM055632-12/GM/NIGMS NIH HHS/United States
- R01 GM055632-10/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous