Amyloid beta mediates memory formation - PubMed (original) (raw)
Amyloid beta mediates memory formation
Ana Garcia-Osta et al. Learn Mem. 2009.
Abstract
The amyloid precursor protein (APP) undergoes sequential cleavages to generate various polypeptides, including the amyloid beta (1-42) peptide (Abeta[1-42]), which is believed to play a major role in amyloid plaque formation in Alzheimer's disease (AD). Here we provide evidence that, in contrast with its pathological role when accumulated, endogenous Abeta in normal hippocampi mediates learning and memory formation. Furthermore, hippocampal injection of picomolar concentrations of exogenous Abeta(1-42) enhances memory consolidation. Correlative data suggest that Abeta peptides may exert their function via nicotinic acethylcoline receptors. Hence, Abeta peptides, including Abeta(1-42), play an important physiological role in hippocampal memory formation.
Figures
Figure 1.
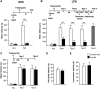
Depletion of endogenous Aβ disrupts memory retention. Memory acquisition (acq) and retention are expressed as mean latency ± SEM (in seconds, s). Rats received intrahippocampal injections of either anti-Aβ or control mAb antibody 15 min before IA training and tested for short- (STM) (A) or long-term memory (LTM) (B) at 1 h or 24 h after training, respectively. Both STM and LTM were disrupted by the anti-Aβ antibody. LTM disruption persisted 5 d after training, and memory did not recover following a reminder foot shock administered in a different context a day later. Amnesic rats that received the anti-Aβ after retraining showed normal retention. ** P < 0.01, *** P < 0.001. (C) Intrahippocampal injections of either anti-Aβ or control mAb immediately after training had no effect on memory tested at 24 h and 5 d after training. (D) No effect of intrahippocampal injections of anti-Aβ on the nociceptive hot plate test. Rats injected with anti-Aβ or control mAb underwent the hot plate test 15 min after injection (Carter 1991). Values are expressed as the mean ± SEM of response latencies measured in seconds. (E) No effect of intrahippocampal injection of anti-Aβ on locomotor activity. One hour after injection of either anti-Aβ or control mAb antibody, rats were allowed to explore the IA training apparatus for 3 min (Roesler et al. 2000) and the locomotor activity was detected by a system of photocell infrared beams. Values are expressed as mean ± SEM of motility counts.
Figure 2.
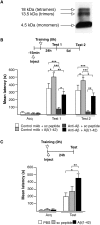
Memory impairment produced by the depletion of endogenous Aβ(1−42) is rescued by exogenous oligomeric human Aβ(1−42). (A) Oligo/monomeric preparation of Aβ42 was examined by 4%–12% _tris_-tricine nondenaturing PAGE Western blotting (Garcia-Osta et al. 2006) using the anti-Aβ monoclonal antibody 6E10 (Covance Research 1:1000) (Tomiyama et al. 2008). Bands corresponding to tetramers, trimers, and monomers were detected. (B) Memory acquisition (Acq) and retention are expressed as mean latency ± SEM (in seconds, s). Rats received intrahippocampal injections of either anti-Aβ or control mAb antibody combined with either scrambled (sc) peptide or Aβ(1–42) 15 min before IA training. *P < 0.05, ** P < 0.01, *** P < 0.001. Test 1, 24 h after training; Test 2, 5 d after training. (C) Memory acquisition (Acq) and retention expressed as mean latency ± SEM (s) of rats that received intrahippocampal injections of PBS, sc peptide, or Aβ(1–42) immediately after IA training. Administration of Aβ(1–42) immediately after IA training enhances memory retention 24 h after training. * P < 0.05, ** P < 0.01.
Figure 3.
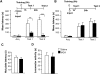
The nicotinic receptor antagonist mecamylamine (MCA) mimics the effect of the anti-Aβ(1–42) antibody. Memory acquisition (Acq) and retention expressed as mean latency ± SEM (in seconds, s) of rats that received intrahippocampal injections of saline or MCA 15 min before IA training (A) or immediately after IA training (B). STM was tested 1 h after training and LTM was tested 24 h after training. ** P < 0.01, *** P < 0.001. (C) Effect of intrahippocampal injection of saline or MCA on nociceptive hot plate test. Rats injected with MCA or saline underwent the hot plate test 15 min after injection (Carter 1991). Values are the mean ± SEM of response latencies measured in seconds. No difference in hot plate latencies was detected between the two groups. (D) Effect of intrahippocampal injection of saline or MCA on locomotor activity. One hour after injection of either saline or MCA rats were tested for locomotor activity as described in Figure 1. Values are the mean ± SEM of motility counts. No difference in locomotor activity was detected between the two groups.
Similar articles
- Amyloid beta peptide levels and its effects on hippocampal acetylcholine release in aged, cognitively-impaired and -unimpaired rats.
Vaucher E, Aumont N, Pearson D, Rowe W, Poirier J, Kar S. Vaucher E, et al. J Chem Neuroanat. 2001 Jun;21(4):323-9. doi: 10.1016/s0891-0618(01)00120-x. J Chem Neuroanat. 2001. PMID: 11429273 - Dexamethasone and Aβ₂₅-₃₅ accelerate learning and memory impairments due to elevate amyloid precursor protein expression and neuronal apoptosis in 12-month male rats.
Li WZ, Li WP, Huang DK, Kan HW, Wang X, Wu WY, Yin YY, Yao YY. Li WZ, et al. Behav Brain Res. 2012 Feb 1;227(1):142-9. doi: 10.1016/j.bbr.2011.10.038. Epub 2011 Oct 29. Behav Brain Res. 2012. PMID: 22061800 - Loss of alpha7 nicotinic receptors enhances beta-amyloid oligomer accumulation, exacerbating early-stage cognitive decline and septohippocampal pathology in a mouse model of Alzheimer's disease.
Hernandez CM, Kayed R, Zheng H, Sweatt JD, Dineley KT. Hernandez CM, et al. J Neurosci. 2010 Feb 17;30(7):2442-53. doi: 10.1523/JNEUROSCI.5038-09.2010. J Neurosci. 2010. PMID: 20164328 Free PMC article. - Treadmill Exercise Ameliorates Spatial Learning and Memory Deficits Through Improving the Clearance of Peripheral and Central Amyloid-Beta Levels.
Khodadadi D, Gharakhanlou R, Naghdi N, Salimi M, Azimi M, Shahed A, Heysieattalab S. Khodadadi D, et al. Neurochem Res. 2018 Aug;43(8):1561-1574. doi: 10.1007/s11064-018-2571-2. Epub 2018 Jun 11. Neurochem Res. 2018. PMID: 29948724 - The many faces of amyloid beta in Alzheimer's disease.
Chiang PK, Lam MA, Luo Y. Chiang PK, et al. Curr Mol Med. 2008 Sep;8(6):580-4. doi: 10.2174/156652408785747951. Curr Mol Med. 2008. PMID: 18781964 Review.
Cited by
- Targeting therapy for homocysteic acid in the blood represents a potential recovery treatment for cognition in Alzheimer's disease patients.
Hasegawa T, Ukai W. Hasegawa T, et al. Aging (Albany NY). 2016 Sep 14;8(9):1838-1843. doi: 10.18632/aging.101046. Aging (Albany NY). 2016. PMID: 27632569 Free PMC article. - Amyloid-β peptide: Dr. Jekyll or Mr. Hyde?
Puzzo D, Arancio O. Puzzo D, et al. J Alzheimers Dis. 2013;33 Suppl 1(0 1):S111-20. doi: 10.3233/JAD-2012-129033. J Alzheimers Dis. 2013. PMID: 22735675 Free PMC article. Review. - Amyloid β Enhances Typical Rodent Behavior While It Impairs Contextual Memory Consolidation.
Salgado-Puga K, Prado-Alcalá RA, Peña-Ortega F. Salgado-Puga K, et al. Behav Neurol. 2015;2015:526912. doi: 10.1155/2015/526912. Epub 2015 Jul 1. Behav Neurol. 2015. PMID: 26229236 Free PMC article. - The physiological roles of tau and Aβ: implications for Alzheimer's disease pathology and therapeutics.
Kent SA, Spires-Jones TL, Durrant CS. Kent SA, et al. Acta Neuropathol. 2020 Oct;140(4):417-447. doi: 10.1007/s00401-020-02196-w. Epub 2020 Jul 29. Acta Neuropathol. 2020. PMID: 32728795 Free PMC article. Review. - Physiological Roles of Monomeric Amyloid-β and Implications for Alzheimer's Disease Therapeutics.
Jeong H, Shin H, Hong S, Kim Y. Jeong H, et al. Exp Neurobiol. 2022 Apr 30;31(2):65-88. doi: 10.5607/en22004. Exp Neurobiol. 2022. PMID: 35673997 Free PMC article. Review.
References
- Allinson T.M., Parkin E.T., Turner A.J., Hooper N.M. ADAMs family members as amyloid precursor protein α-secretases. J. Neurosci. Res. 2003;74:342–352. - PubMed
- Canal C.E., Gold P.E. Different temporal profiles of amnesia after intra-hippocampus and intra-amygdala infusions of anisomycin. Behav. Neurosci. 2007;121:732–741. - PubMed
- Carter R.B. Differentiating analgesic and non-analgesic drug activities on rat hot plate: Effect of behavioral endpoint. Pain. 1991;47:211–220. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical