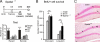Recruitment of adult-generated neurons into functional hippocampal networks contributes to updating and strengthening of spatial memory - PubMed (original) (raw)
Recruitment of adult-generated neurons into functional hippocampal networks contributes to updating and strengthening of spatial memory
Stéphanie Trouche et al. Proc Natl Acad Sci U S A. 2009.
Abstract
The dentate gyrus (DG), a hippocampal subregion, continuously produces new neurons in the adult mammalian brain that become functionally integrated into existing neural circuits. To what extent this form of plasticity contributes to memory functions remains to be elucidated. Using mapping of activity-dependent gene expression, we visualized in mice injected with the birthdating marker 5-bromo-2'-deoxyuridine the recruitment of new neurons in a set of controlled water maze procedures that engage specific spatial memory processes and require hippocampal-cortical networks. Here, we provide new evidence that adult-generated hippocampal neurons make a specific but differential contribution to the processing of remote spatial memories. First, we show that new neurons in the DG are recruited into neuronal networks that support retrieval of remote spatial memory and that their activation is situation-specific. We further reveal that once selected, new hippocampal neurons are durably incorporated into memory circuits, and also that their recruitment into hippocampal networks contributes predominantly to the updating and strengthening of a previously encoded memory. We find that initial spatial training during a critical period, when new neurons are more receptive to surrounding neuronal activity, favors their subsequent recruitment upon remote memory retrieval. We therefore hypothesize that new neurons activated during this critical period become tagged so that once mature, they are preferentially recruited into hippocampal networks underlying remote spatial memory representation when encountering a similar experience.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Spatial learning promotes survival of new hippocampal cells generated 9 days before training. (A) Spatially trained mice (Spatial−PF) received 3 injections of BrdU (arrows) on day 1 (D1) and were submitted to acquisition of the hidden version of the Morris water maze on day 9 (D9). Mice were tested for recent or remote memory 1 day (D10) or 30 days (D39), respectively, after acquisition during a single probe test held without platform. Numbers of annulus crossings indicate that at both delays, Spatial−PF mice showed a similar preference for the target zone where the platform was located during training compared with the adjacent (A1 and A2) and opposite (Opp.) zones (target vs. others; ***, P < 0.001). No significant forgetting occurred over time. (B) Total number of surviving BrdU-labeled (BrdU+) cells 1 day or 30 days after training when BrdU+ cells were 10 or 39 days old in Spatial−PF compared with Swim and Cage control mice (*, P < 0.05; **, P < 0.01). (C) Representative photomicrographs of sections counterstained with Nuclear Fast Red showing adult-generated granule cells visualized by BrdU immunostaining (arrows) in the dentate gyrus of Swim (Upper) and Spatial−PF (Lower) animals tested 30 days after spatial learning. (Scale bar: 100 μm.)
Fig. 2.
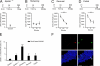
Retrieval of remote spatial memory activates the dentate gyrus. (A) Densities of Zif268+ nuclei were higher in the dentate gyrus of Spatial−PF mice compared with Swim and Cage control mice after testing for remote memory (***, P < 0.001; *, P < 0.05). (B) Photomicrographs showing Zif268 staining in the DG of Swim and Spatial−PF mice. (Scale bar: 150 μm.)
Fig. 3.
Recruitment of adult-generated neurons in the dentate gyrus is situation-specific. (A) Spatial+PF mice were submitted to training on day 9 (D9) and tested for remote memory on day 39 in the presence of the hidden platform. During the remote trial, Spatial+PF animals exhibited swim path lengths similar to those observed at the end of training (
Fig. S5
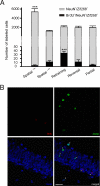
), indicating no memory forgetting. (B) Retraining mice trained on day 9 were submitted to 3 blocks of 3 trials of spatial training on day 39 in the presence of the hidden platform. During retraining on day 39 (blocks 9–11), performances remained at an asymptotic level. (C) Mice trained on day 9 were submitted to a reversal paradigm on day 39, with the hidden platform present at a new location (Reversal group). As shown by their decreasing swim path length to the hidden platform, Reversal mice quickly learned the new platform position (**, P < 0.01). (D) Mice from the Partial group remained in their home cage until they were trained in the water maze on day 39. Over the 3 blocks of 3 trials, mice from this Partial group exhibited decreased swim path length, indicating that they started to learn the location of the hidden platform (**, P < 0.01). (E) Proportion of new neurons (BrdU+/NeuN+ cells) expressing Zif268 after remote memory testing in the Spatial−PF, Spatial+PF, Retraining, Reversal, and Partial conditions. A significantly higher proportion of activated new neurons (triple-labeled BrdU+/NeuN+/Zif268+ cells) was found in the Retraining group upon testing on day 39 compared with the other groups (***, P < 0.001). (F) High-magnification confocal images depicting populations of activated Zif268+ cells (green), BrdU+ cells (red), and granular neurons labeled with NeuN (blue) in the DG of a Retraining mouse. Arrows in the merged picture indicate NeuN+ cells coexpressing Zif268, and arrowhead identifies a triple-labeled BrdU+/NeuN+/Zif268+ neuron. (Scale bar: 20 μm.)
Fig. 4.
Recruitment of new neurons in the dentate gyrus is modulated by the experimental constraints. (A) Numbers of new neurons (BrdU+/NeuN+/Zif268+) that contribute to the overall neuronal activation (NeuN+/ Zif268+) in the DG across the different conditions of memory testing (NeuN+/Zif268+: ***, P < 0.001 vs. other conditions; BrdU+/NeuN+/Zif268+: ***, P < 0.001 vs. other conditions). (B) Representative confocal images depicting 39-day-old BrdU+ cells (red), Zif268+ activated cells (green), and NeuN+ mature neurons (blue) in the DG of a mouse submitted to reversal training. Arrows in the merged picture indicate NeuN-labeled cell coexpressing Zif268, and arrowheads identify BrdU+ cells coexpressing NeuN. (Scale bar: 20 μm.)
Similar articles
- Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus.
Kee N, Teixeira CM, Wang AH, Frankland PW. Kee N, et al. Nat Neurosci. 2007 Mar;10(3):355-62. doi: 10.1038/nn1847. Epub 2007 Feb 4. Nat Neurosci. 2007. PMID: 17277773 - Young hippocampal neurons are critical for recent and remote spatial memory in adult mice.
Goodman T, Trouche S, Massou I, Verret L, Zerwas M, Roullet P, Rampon C. Goodman T, et al. Neuroscience. 2010 Dec 15;171(3):769-78. doi: 10.1016/j.neuroscience.2010.09.047. Epub 2010 Sep 29. Neuroscience. 2010. PMID: 20883747 - The timing of differentiation of adult hippocampal neurons is crucial for spatial memory.
Farioli-Vecchioli S, Saraulli D, Costanzi M, Pacioni S, Cinà I, Aceti M, Micheli L, Bacci A, Cestari V, Tirone F. Farioli-Vecchioli S, et al. PLoS Biol. 2008 Oct 7;6(10):e246. doi: 10.1371/journal.pbio.0060246. PLoS Biol. 2008. PMID: 18842068 Free PMC article. - Adult hippocampal neurogenesis, synaptic plasticity and memory: facts and hypotheses.
Bruel-Jungerman E, Rampon C, Laroche S. Bruel-Jungerman E, et al. Rev Neurosci. 2007;18(2):93-114. doi: 10.1515/revneuro.2007.18.2.93. Rev Neurosci. 2007. PMID: 17593874 Review. - Review: adult neurogenesis contributes to hippocampal plasticity.
Toda T, Gage FH. Toda T, et al. Cell Tissue Res. 2018 Sep;373(3):693-709. doi: 10.1007/s00441-017-2735-4. Epub 2017 Nov 29. Cell Tissue Res. 2018. PMID: 29185071 Review.
Cited by
- Age-dependent role for Ras-GRF1 in the late stages of adult neurogenesis in the dentate gyrus.
Darcy MJ, Trouche S, Jin SX, Feig LA. Darcy MJ, et al. Hippocampus. 2014 Mar;24(3):315-25. doi: 10.1002/hipo.22225. Hippocampus. 2014. PMID: 24174283 Free PMC article. - Stimulation of entorhinal cortex promotes adult neurogenesis and facilitates spatial memory.
Stone SS, Teixeira CM, Devito LM, Zaslavsky K, Josselyn SA, Lozano AM, Frankland PW. Stone SS, et al. J Neurosci. 2011 Sep 21;31(38):13469-84. doi: 10.1523/JNEUROSCI.3100-11.2011. J Neurosci. 2011. PMID: 21940440 Free PMC article. - Primary cilia regulate proliferation of amplifying progenitors in adult hippocampus: implications for learning and memory.
Amador-Arjona A, Elliott J, Miller A, Ginbey A, Pazour GJ, Enikolopov G, Roberts AJ, Terskikh AV. Amador-Arjona A, et al. J Neurosci. 2011 Jul 6;31(27):9933-44. doi: 10.1523/JNEUROSCI.1062-11.2011. J Neurosci. 2011. PMID: 21734285 Free PMC article. - Hippocampal adult neurogenesis: Its regulation and potential role in spatial learning and memory.
Lieberwirth C, Pan Y, Liu Y, Zhang Z, Wang Z. Lieberwirth C, et al. Brain Res. 2016 Aug 1;1644:127-40. doi: 10.1016/j.brainres.2016.05.015. Epub 2016 May 10. Brain Res. 2016. PMID: 27174001 Free PMC article. Review. - Doxycycline increases neurogenesis and reduces microglia in the adult hippocampus.
Sultan S, Gebara E, Toni N. Sultan S, et al. Front Neurosci. 2013 Jul 25;7:131. doi: 10.3389/fnins.2013.00131. eCollection 2013. Front Neurosci. 2013. PMID: 23898238 Free PMC article.
References
- Shors TJ, et al. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. - PubMed
- Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, Wang S. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus. 2006;16:296–304. - PubMed
- Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130:843–852. - PubMed
- Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007;10:355–362. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases