E-Cadherin regulates neural stem cell self-renewal - PubMed (original) (raw)
E-Cadherin regulates neural stem cell self-renewal
Phillip Karpowicz et al. J Neurosci. 2009.
Abstract
E-Cadherin, a cell adhesion protein, has been shown to take part in the compartmentalization, proliferation, survival, and differentiation of cells. E-Cadherin is expressed in the adult and embryonic forebrain germinal zones in vivo, and in clonal colonies of cells derived from these regions and grown in vitro. Mice carrying E-Cadherin floxed genes crossed to mice expressing Cre under the Nestin promoter demonstrate defects in the self-renewal of neural stem cells both in vivo and in vitro. The functional role of E-Cadherin is further demonstrated using adhesion-blocking antibodies in vitro, which specifically target cadherin extracellular adhesive domains. Adult neural stem cell colonies decrease in the presence of E-Cadherin antibodies in a dosage-dependent manner, in contrast to P-Cadherin antibody. On overexpression of normal E-Cadherin and a mutated E-Cadherin, containing no intracellular binding domain, an increased number of clonal adult neural stem cell colonies are observed. These data suggest it is specifically E-Cadherin adhesion that is responsible for these self-renewal effects. These data show the importance of E-Cadherin in the neural stem cell niche and suggest E-Cadherin regulates the number of these cells.
Figures
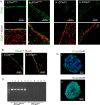
Figure 1.
A, E-Cadherin is expressed in the adult murine ventricles. Image shows confocal micrographs of E-Cadherin in the adult forebrain lateral ventricular germinal zone (green). E-Cadherin stains primarily in ependymal cells and to a lesser extent in subependymal cells in wild-type animals (i, ii) versus conditional knock-outs (iii, iv), whose E-Cadherin staining is almost completely absent. Nuclei are shown in merge in red. B, N-Cadherin is expressed in the adult murine ventricles. The image shows confocal micrographs of N-Cadherin in the forebrain lateral ventricular germinal zone (green). Similar to E-Cadherin, N-Cadherin staining is seen particularly in the ependymal cells rather than subependymal cells. Both E-Cadherin wild type (i) and conditional knock-outs (ii) display N-Cadherin in these regions. Nuclei are shown in merge in red. C, E-Cadherin and N-Cadherin are expressed at day 7 in vitro in adult NSC colonies. Merged images show sections of large NSC colonies stained for E-Cadherin (green; i) and N-Cadherin (green; ii). DAPI (4′,6-diamidino-2-phenylindole) counterstain is blue. N-Cadherin staining was noted to be stronger and more widely expressed than E-Cadherin in these colonies. D, E-Cadherin PCR of colonies derived from NSCs dissected from adult forebrain ventricles. Image shows complete loss of E-Cadherin PCR product in wild-type (n = 5 animals; rows 1–5) versus conditional knock-out animals (n = 5 animals; rows 6–10). DNA was extracted from passage 2 NSC colonies to avoid contamination from endothelial cells, also present in the SVZ, which express E-Cadherin protein. Because primers span intron 10 of the E-Cadherin gene, the larger band indicates alleles in which a loxP site is present. The lower bands show wild-type allele. Row 11 is negative (water) control.
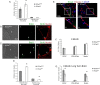
Figure 2.
A, BrdU uptake is increased but BrdU retention is equivalent, in 2 month E-Cadherin conditional knock-out forebrain ventricular subependyma. Left, The graph shows an increase in BrdU+ progenitors in EcadΔ/Δ mice (n = 3) compared with their EcadWt/Wt littermate controls (n = 3) at 2 months of age. The asterisk indicates that the difference is significant (t = 4.529; df = 58; p < 0.05). Right, Same graph shows numbers of BrdU+ nuclei in 2-month-old BrdU+ retaining progenitors, 1 month after BrdU injection, in EcadΔ/Δ mice (n = 3) compared with their EcadWt/Wt littermate controls (n = 3). There are no significant differences. B, BrdU uptake in 9 month wild-type forebrain ventricles. The sections show BrdU-positive EcadWt/Wt neural precursors (arrow) after 1 h BrdU exposure in vivo. i shows brightfield. BrdU is green (ii, iii) and nuclei are counterstained in red by a pan-histone antibody (iii). C, BrdU uptake in 9 month E-Cadherin conditional knock-out forebrain ventricles. Sections show BrdU EcadΔ/Δ-positive neural precursors (arrows) after 1 h BrdU exposure in vivo. i shows brightfield. BrdU is green (ii, iii) and nuclei are red (iii). Note the increase in EcadΔ/Δ cells entering S-phase during short-term BrdU administration. D, BrdU uptake is increased but BrdU retention is decreased in 9 month E-Cadherin conditional knock-out forebrain ventricular subependyma. Left, The graph shows increase in BrdU+ progenitors in EcadΔ/Δ mice (n = 4) compared with their EcadWt/Wt littermate controls (n = 5) at 9 months of age. The asterisk indicates that the difference in BrdU+ nuclei after 1 h BrdU exposure is significant (t = 6.013; df = 88; p < 0.05). Right, Same graph shows decrease in 9-month-old BrdU+ retaining progenitors, 1 month after BrdU administration, in EcadΔ/Δ mice (n = 5) compared with their EcadWt/Wt littermate controls (n = 5). The asterisk indicates this reduction is significant (t = 3.899; df = 118; p < 0.05) in contrast to that observed in 2 month animals. E, GFAP is present in both wild-type and conditional knock-out forebrain ventricles. Confocal micrographs show GFAP positivity in both EcadWt/Wt (i, iii) as well as EcadΔ/Δ (ii, iv) SVZ cells. E-Cadherin is green, GFAP is blue, and nuclei are counterstained red. The boxed area in i is shown magnified in iii, and ii in iv. F, E-Cadherin conditional knock-out forebrain shows normal cell demographics. The graph shows numbers of GFAP, PSA-NCam, and Nestin-positive cells present in the 9-month-old forebrain of EcadWt/Wt and EcadΔ/Δ animals. No significant differences are noted in the presence of any of these cell types (n = 3 animals of each genotype sampled). G, E-Cadherin loss leads to reduction in GFAP and Nestin BrdU-retaining cells in 9-month-old animals. The graph shows numbers of BrdU-retaining cells that colabel for GFAP, PSA-NCam, or Nestin. The asterisks show that there are significant differences between EcadWt/Wt and EcadΔ/Δ GFAP-expressing cells (t = 5.758; df = 4; p < 0.05) as well as Nestin-expressing cells (t = 4.341; df = 4; p < 0.05). These results contrast with the generally equivalent labeling of GFAP and Nestin in the aged forebrain and suggest the decreases in these numbers are specific to the BrdU-retaining cell population. Error bars indicate SEM.
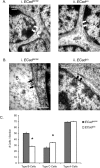
Figure 3.
A, Adherent junctions between type A and B cells are present in the 9 month E-Cadherin conditional knock-out subependyma. Shown are electron micrographs from EcadWt/Wt (i) and EcadΔ/Δ (ii) animals. As in wild-type cells, protein dense adherent junctions (arrows) were identified in conditional mutants, despite the loss of E-Cadherin in these cells. This may be attributable to the presence of N-Cadherin, which is highly expressed in these cell types. “A” indicates type A cell, and “B” indicates type B cell. B, EcadΔ/Δ B cells contact ependymal cells normally in the 9 month E-Cadherin conditional knock-out subependyma. Electron micrographs were examined in EcadWt/Wt (i) and EcadΔ/Δ (ii) animals. It was found that EcadΔ/Δ B cells contacted ependymal cells similar to EcadWt/Wt (arrows). “B” indicates type B cell, and “E” indicates type E cell. No differences were noted in these structures between wild types and conditional mutants. C, E-Cadherin loss leads to reduction in B cells and increase in C cells in the 9 month E-Cadherin conditional knock-out subependyma. The graph shows numbers of cell types identified by electron microscopy in the subependymal zone. Although there are no differences in the number of type A cells, EcadΔ/Δ mice show significant differences (asterisks) between B cells (t = 9.594; df = 38; p < 0.05) and C cells (t = 6.584; df = 38; p < 0.05). Counts are expressed per section; 20 sections were sampled from n = 3 animals of each type, with at least six sections sampled per animal. Error bars indicate SEM.
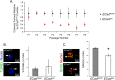
Figure 4.
A, NSC colony formation is reduced in E-Cadherin conditional knock-outs. The graph shows the inability of EcadΔ/Δ NSCs to maintain over long-term passage, in contrast to EcadWt/Wt littermate controls. Clonal NSC colonies were averaged arising from every 5000 cells plated on dissection, or 2500 cells plated after passage. The averages from EcadΔ/Δ were normalized to the EcadWt/Wt controls. Colonies of EcadΔ/Δ begin to show a deficit in number at passage 3 after dissection (P3) and maintain this deficit thereafter. The remaining colonies were passaged exactly as wild types; both were replated in 24-well plates. Differences between EcadWt/Wt and EcadΔ/Δ are significant at passages 3 onward, and the interaction between genotype and passage is significant (F(15,109) = 1.946; p < 0.05). Primary time point is an average of cells dissected from _n_ = 10 (EcadΔ/Δ) and _n_ = 11 (EcadWt/Wt) animals; the remaining time points contain _n_ = 7 mice per passage EcadΔ/Δ and _n_ = 4–6 mice per passage EcadWt/Wt. **_B_**, Cell death is equivalent between wild-type and E-Cadherin conditional knock-out colonies. Images show merge of 3 d EcadΔ/Δ and EcadWt/Wt colonies at passage 3, the time point at which differences in colony number arise between these groups. TUNEL assay (arrow) reveals cell nuclei undergoing apoptosis (green); nuclei are counterstained by DAPI (blue). The graph shows that there are no significant differences (_p_ > 0.05) between the proportion of TUNEL+ cells per colony (n = 3 animals per group). C, Proliferation is decreased in E-Cadherin conditional knock-out colonies at passage 3. Images show merge of 3 d EcadΔ/Δ and EcadWt/Wt colonies at passage 3, the time point at which differences in colony number arise between these groups. BrdU uptake (arrow) reveals nuclei that have entered S-phase (yellow in merged image). Nuclei are counterstained using pan-histone (red). The graph shows that there are significant differences (asterisk) between the proportion of BrdU+ cells per colony (t = 4.043; df = 4; p < 0.05; n = 3 animals per group). Error bars indicate SEM.
Figure 5.
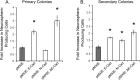
A, E-Cadherin antibodies reduce NSC colony formation in a dose-dependent manner. The graph shows decrease in number of primary colonies as adhesion blocking α-Cadherin antibody concentration increases. Colony formation in α-P-Cadherin antibody does not vary at any concentration (p > 0.05). The interaction between antibody and dosage is significant (F(153,57) = 15.75; p < 0.05). Multiple-comparison tests show that, at 1.0 μg/ml concentration, ECCD-1 and α-N-Cadherin antibody induce a significant decrease in the number of colonies relative to α-P-Cadherin. At 2.0 μg/ml concentration, the numbers of colonies with all three antibodies (ECCD-1, ECCD-2, and α-N-Cadherin) are significantly lower than α-P-Cadherin. B**, Secondary NSC colony formation is only affected by E-Cadherin adhesion block. Secondary colonies were grown in the absence of antibody. The graph shows a decrease in colony number (asterisk) observed in colonies subcloned from cells exposed to 1.0 μg/ml ECCD-1 antibody but not α-N-Cadherin or α-P-Cadherin during primary colony formation. An ANOVA test shows main effect of antibody (F(153,84) = 17.14; p < 0.05), and multiple-comparison tests show that only ECCD-1 is different from control cells never exposed to antibody. **C_**, Tertiary NSC colony formation is normal. The graph shows recovery in colony formation observed in tertiary spheres grown for two passages, in the absence of antibody, after exposure to 1.0 μg/ml during primary colony formation. Cells never exposed to antibody produce similar numbers of spheres as any of the antibody-exposed groups. An ANOVA shows there are no significant differences between any of the groups tested (F(153,72) = 2.16; p > 0.05). D, Antibody exposure at day 3 of colony formation does not affect colony number. The graph shows that, unlike the decreases observed when plating cells directly into α-Cadherin antibodies (Fig. 3_A,B), there are no differences among antibodies when the antibodies are applied at 1.0 μg/ml at day 3 in vitro (F(152,14) = 0.30; p > 0.05). These results suggest colony decreases are attributable to antibody effects during the first 3 d. E, Adhesion block does not induce cell toxicity. The graph shows that exposure to antibodies at 1.0 μg/ml does not influence cell death. In no case did antibodies increase cell death over controls plated in the absence of antibody (F(154,24) = 1.33; p > 0.05). F, α-E-Cadherin affects the differentiated progeny of NSCs. Images show colonies of NSCs grown and differentiated in the presence of ECCD-1 (i) or α-P-Cadherin (ii) antibodies. Proteins of interest are shown in red; nuclei are counterstained in blue (DAPI). Note the obvious altered morphology and number of neurons (β-III Tubulin+) and astrocytes (GFAP+) types grown in ECCD-1 as opposed to α-P-Cadherin control antibody. G, Neuron and astrocyte number is reduced under conditions of E-Cadherin and N-Cadherin adhesion block. The graphs show reduction in neuronal production (n = 4 colonies sampled; main effect of antibody, F(153,13) = 9.831, p < 0.05) (i) and astrocyte production (n = 4 colonies sampled; main effect of antibody, F(153,15) = 15.89, p < 0.05) (ii**) by NSC colonies. Colonies were both grown and differentiated in presence of antibody. The asterisks indicate groups that are significantly different from α-P-Cadherin control in multiple-comparison tests. Error bars indicate SEM.
Figure 6.
A, E-Cadherin increases primary clonal NSC colony formation. The graph shows an increase in neurosphere production when NSCs are induced to overexpress E-Cadherin. ANOVA indicates main effect of vector (F(153,207) = 29.93; p < 0.05). The asterisks indicate pMXIE:E-Cad and pMXIE:ΔE-Cad significantly increase colony number over control pMXIE. B, E-Cadherin and N-Cadherin increase secondary clonal NSC colony formation. Clones from A were passaged to assess overexpression effect on secondary colony formation. The graph shows increase in number of colonies on passage, after E- or N-Cadherin overexpression. ANOVA test shows main effect of vector (F(153,177) = 31.03; p < 0.05). The asterisks indicate pMXIE:E-Cad, pMXIE:N-Cad, and pMXIE:ΔE-Cad are all significantly higher than control pMXIE. Error bars indicate SEM.
Similar articles
- USP9X enhances the polarity and self-renewal of embryonic stem cell-derived neural progenitors.
Jolly LA, Taylor V, Wood SA. Jolly LA, et al. Mol Biol Cell. 2009 Apr;20(7):2015-29. doi: 10.1091/mbc.e08-06-0596. Epub 2009 Jan 28. Mol Biol Cell. 2009. PMID: 19176755 Free PMC article. - SOX2, a persistent marker for multipotential neural stem cells derived from embryonic stem cells, the embryo or the adult.
Ellis P, Fagan BM, Magness ST, Hutton S, Taranova O, Hayashi S, McMahon A, Rao M, Pevny L. Ellis P, et al. Dev Neurosci. 2004 Mar-Aug;26(2-4):148-65. doi: 10.1159/000082134. Dev Neurosci. 2004. PMID: 15711057 - Abrogation of E-cadherin-mediated cell-cell contact in mouse embryonic stem cells results in reversible LIF-independent self-renewal.
Soncin F, Mohamet L, Eckardt D, Ritson S, Eastham AM, Bobola N, Russell A, Davies S, Kemler R, Merry CL, Ward CM. Soncin F, et al. Stem Cells. 2009 Sep;27(9):2069-80. doi: 10.1002/stem.134. Stem Cells. 2009. PMID: 19544408 - Characterization and neural differentiation of mouse embryonic and induced pluripotent stem cells on cadherin-based substrata.
Haque A, Yue XS, Motazedian A, Tagawa Y, Akaike T. Haque A, et al. Biomaterials. 2012 Jul;33(20):5094-106. doi: 10.1016/j.biomaterials.2012.04.003. Epub 2012 Apr 19. Biomaterials. 2012. PMID: 22520296 - Stem cells use distinct self-renewal programs at different ages.
Levi BP, Morrison SJ. Levi BP, et al. Cold Spring Harb Symp Quant Biol. 2008;73:539-53. doi: 10.1101/sqb.2008.73.049. Epub 2009 Jan 15. Cold Spring Harb Symp Quant Biol. 2008. PMID: 19150957 Review.
Cited by
- Peripheral Delivery of Neural Precursor Cells Ameliorates Parkinson's Disease-Associated Pathology.
Edwards Iii G, Gamez N, Armijo E, Kramm C, Morales R, Taylor-Presse K, Schulz PE, Soto C, Moreno-Gonzalez I. Edwards Iii G, et al. Cells. 2019 Oct 30;8(11):1359. doi: 10.3390/cells8111359. Cells. 2019. PMID: 31671704 Free PMC article. - Neural-Cadherin Influences the Homing of Terminally Differentiated Memory CD8 T Cells to the Lymph Nodes and Bone Marrow.
Kim KH, Choi A, Kim SH, Song H, Jin S, Kim K, Jang J, Choi H, Jung YW. Kim KH, et al. Mol Cells. 2021 Nov 30;44(11):795-804. doi: 10.14348/molcells.2021.0137. Mol Cells. 2021. PMID: 34819396 Free PMC article. - Tissue-resident memory T cells.
Shin H, Iwasaki A. Shin H, et al. Immunol Rev. 2013 Sep;255(1):165-81. doi: 10.1111/imr.12087. Immunol Rev. 2013. PMID: 23947354 Free PMC article. Review. - Cdh1 functions as an oncogene by inducing self-renewal of lung cancer stem-like cells via oncogenic pathways.
Ye T, Li J, Sun Z, Liu D, Zeng B, Zhao Q, Wang J, Xing HR. Ye T, et al. Int J Biol Sci. 2020 Jan 1;16(3):447-459. doi: 10.7150/ijbs.38672. eCollection 2020. Int J Biol Sci. 2020. PMID: 32015681 Free PMC article. - Neuroblast niche position is controlled by Phosphoinositide 3-kinase-dependent DE-Cadherin adhesion.
Doyle SE, Pahl MC, Siller KH, Ardiff L, Siegrist SE. Doyle SE, et al. Development. 2017 Mar 1;144(5):820-829. doi: 10.1242/dev.136713. Epub 2017 Jan 26. Development. 2017. PMID: 28126840 Free PMC article.
References
- Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. - PubMed
- Alvarez-Buylla A, García-Verdugo JM, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci. 2001;2:287–293. - PubMed
- Bendall SC, Stewart MH, Menendez P, George D, Vijayaragavan K, Werbowetski-Ogilvie T, Ramos-Mejia V, Rouleau A, Yang J, Bossé M, Lajoie G, Bhatia M. IGF and FGF cooperatively establish the regulatory stem cell niche of pluripotent human cells in vitro. Nature. 2007;448:1015–1021. - PubMed
- Bendel-Stenzel MR, Gomperts M, Anderson R, Heasman J, Wylie C. The role of cadherins during primordial germ cell migration and early gonad formation in the mouse. Mech Dev. 2000;91:143–152. - PubMed
- Betschinger J, Knoblich JA. Dare to be different: asymmetric cell division in Drosophila, C. elegans and vertebrates. Curr Biol. 2004;14:R674–R685. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous