TEAD transcription factors mediate the function of TAZ in cell growth and epithelial-mesenchymal transition - PubMed (original) (raw)
TEAD transcription factors mediate the function of TAZ in cell growth and epithelial-mesenchymal transition
Heng Zhang et al. J Biol Chem. 2009.
Erratum in
- Correction: TEAD transcription factors mediate the function of TAZ in cell growth and epithelial-mesenchymal transition.
Zhang H, Liu CY, Zha ZY, Zhao B, Yao J, Zhao S, Xiong Y, Lei QY, Guan KL. Zhang H, et al. J Biol Chem. 2019 Apr 12;294(15):5808. doi: 10.1074/jbc.AAC119.008436. J Biol Chem. 2019. PMID: 30979850 Free PMC article. No abstract available.
Abstract
The TAZ transcription co-activator has been shown to promote cell proliferation and to induce epithelial-mesenchymal transition. Recently we have demonstrated that TAZ is phosphorylated and inhibited by the Hippo tumor suppressor pathway, which is altered in human cancer. The mechanism of TAZ-mediated transcription is unclear. We demonstrate here that TEAD is a key downstream transcription factor mediating the function of TAZ. Disruption of TEAD-TAZ binding or silencing of TEAD expression blocked the function of TAZ to promote cell proliferation and to induce epithelial-mesenchymal transition, demonstrating TEAD as a key downstream effector of TAZ. We also identified CTGF, a gene that regulates cell adhesion, proliferation, and migration, as a direct target of TAZ and TEAD. Our study establishes a functional partnership between TAZ and TEAD under negative regulation by the Hippo signaling pathway.
Figures
FIGURE 1.
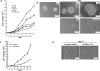
TEADs are the major TAZ binding transcription factors. A, SBP-TAZ is stably expressed in 293T cells. Retrovirus was used to establish SBP-TAZ stable cell clones. SBP-TAZ was purified with streptavidin-Sepharose, and the purified proteins were examined by Western blotting. B, silver staining for the streptavidin-Sepharose purified proteins. The streptavidin-Sepharose purified proteins were separated by SDS-PAGE for silver staining. C, identification of multiple TEAD peptides from TAZ immunocomplex. After SBP purification, trypsin digestion was performed on beads, and the total digestion mixture was directly analyzed by liquid chromatography-MS/MS. The identified peptides for each TEAD family member were shown. D, endogenous TEAD4 was coprecipitated by SBP-TAZ. Cell lysate was precipitated with SBP-Sepharose followed by Western blotting with antibodies against TAED4 and TAZ.
FIGURE 2.
TEAD mediates TAZ-dependent gene induction. A, TAZ activates TEAD family transcription factors. 5× upstream activating sequence-Luciferase reporter, the indicated Gal4-fused transcription factors, and renilla were co-transfected with or without TAZ. The renilla activity normalized luciferase activity in the absence of TAZ was set to 1. B, Ser-51 of TAZ disrupts its interaction with TEAD4. The indicated plasmids were cotransfected into 293T cells. FLAG-TAZ was immunoprecipitated (IP) and probed with the indicated antibodies. C, TAZS51A loses the ability to activate TEAD4. The indicated plasmids were co-transfected with a 5× upstream activating sequence-luciferase reporter into 293T cells. Luciferase activity was measured and normalized to renilla activity.WT, wild type. D, dominant-negative TEAD1 blocks TAZ-mediated activation of CTGF promoter. The indicated plasmids were coexpressed in 293T cells. Cells were harvested for measurement of luciferase activity 24 h after transfection. E, TAZ binds to CTGF promoter. A ChIP assay was performed with anti-FLAG antibody using 293T cells stably expressing FLAG-TAZ. The presence of CTGF promoter was detected by PCR.WT, wild type. F, TEAD is required for TAZ-induced CTGF induction. RNA was extracted from the indicated MCF10A stable pools, and CTGF mRNA levels were analyzed by quantitative RT-PCR.
FIGURE 3.
Disruption of TAZ-TEAD binding abolishes the function of TAZ in promoting cell proliferation. A, growth curve of MCF10A cells stably expressing wild-type or mutant TAZ as indicated. B, TAZ4SA-S51A is compromised in inducing enlarged MCF10A cell acini. Indicated MCF10A-derived stable cells were cultured in three-dimensions on reconstituted basement membrane for 14 days before the pictures were taken.WT, wild type. C, growth curve of TAZ4SA expressing MCF10A cells. TEAD1/3/4 knockdown by shRNA are indicated.D, TEAD knockdown inhibits MCF10A cell acinar growth. TAZ4SA-expressing MCF10A cells were infected with control or TEAD1/3/4 shRNA lentivirus. Cell growth in Matrigel is shown.
FIGURE 4.
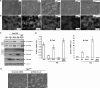
Disruption of TEAD-TAZ binding blocks the ability of TAZ to promote EMT. A, S51A mutation in TAZ blocks TAZ induced EMT-like morphological change. Phase-contrast images of MCF10A cells stably expressing TAZ and its mutants are shown. B, Ser-51 mutation abolishes actin organization remodeling induced by TAZ. Cells were stained with rhodamine-conjugated phalloidin. C, Ser-51 mutation abolishes TAZ-induced EMT. Cell lysates from MCF10A cells stably expressing the wild-type (WT) and mutant TAZ were analyzed with the indicated epithelial and mesenchymal marker antibodies. D, Ser-51 mutation abolishes TAZ-induced change of E-cadherin and N-cadherin mRNA expression. Total RNA was extracted from MCF10A cells stably expressing the wild-type and mutant TAZ, and quantitative RT-PCR was employed to determine E-cadherin and N-cadherin mRNA levels. E, TEAD1/3/4 knockdown blocks TAZ-induced EMT-like morphological change. TEAD1/3/4 were knocked down by shRNA. Indicated MCF10A stable cells were cultured, and the pictures were taken.
FIGURE 5.
TEAD is required for TAZ function in promoting cell migration. A, Ser-51 mutation abolishes TAZ-induced cell migration. Migration of MCF10A cells stably expressing the wild-type and mutant TAZ was determined by a wound-healing assay. B, TEAD1/3/4 knockdown blocks TAZ induced cell migration. Migration ability of indicated MCF10A stable cells was determined by a wound-healing assay.
Similar articles
- Hippo Component TAZ Functions as a Co-repressor and Negatively Regulates ΔNp63 Transcription through TEA Domain (TEAD) Transcription Factor.
Valencia-Sama I, Zhao Y, Lai D, Janse van Rensburg HJ, Hao Y, Yang X. Valencia-Sama I, et al. J Biol Chem. 2015 Jul 3;290(27):16906-17. doi: 10.1074/jbc.M115.642363. Epub 2015 May 20. J Biol Chem. 2015. PMID: 25995450 Free PMC article. - Cysteine S-Glutathionylation Promotes Stability and Activation of the Hippo Downstream Effector Transcriptional Co-activator with PDZ-binding Motif (TAZ).
Gandhirajan RK, Jain M, Walla B, Johnsen M, Bartram MP, Huynh Anh M, Rinschen MM, Benzing T, Schermer B. Gandhirajan RK, et al. J Biol Chem. 2016 May 27;291(22):11596-607. doi: 10.1074/jbc.M115.712539. Epub 2016 Apr 5. J Biol Chem. 2016. PMID: 27048650 Free PMC article. - Effects of TAZ on human dental pulp stem cell proliferation and migration.
Tian S, Tian X, Liu Y, Dong F, Wang J, Liu X, Zhang Z, Chen H. Tian S, et al. Mol Med Rep. 2017 Jun;15(6):4326-4332. doi: 10.3892/mmr.2017.6550. Epub 2017 May 3. Mol Med Rep. 2017. PMID: 28487958 - Roles of RUNX in Hippo Pathway Signaling.
Passaniti A, Brusgard JL, Qiao Y, Sudol M, Finch-Edmondson M. Passaniti A, et al. Adv Exp Med Biol. 2017;962:435-448. doi: 10.1007/978-981-10-3233-2_26. Adv Exp Med Biol. 2017. PMID: 28299672 Free PMC article. Review. - Hippo pathway inhibition by blocking the YAP/TAZ-TEAD interface: a patent review.
Crawford JJ, Bronner SM, Zbieg JR. Crawford JJ, et al. Expert Opin Ther Pat. 2018 Dec;28(12):867-873. doi: 10.1080/13543776.2018.1549226. Epub 2018 Dec 2. Expert Opin Ther Pat. 2018. PMID: 30482112 Review.
Cited by
- Hepatic epithelioid hemangioendothelioma and the danger of misdiagnosis: report of a case.
Neofytou K, Chrysochos A, Charalambous N, Dietis M, Petridis C, Andreou C, Petrou A. Neofytou K, et al. Case Rep Oncol Med. 2013;2013:243939. doi: 10.1155/2013/243939. Epub 2013 Feb 28. Case Rep Oncol Med. 2013. PMID: 23533870 Free PMC article. - Vascular bone tumors: a proposal of a classification based on clinicopathological, radiographic and genetic features.
Errani C, Vanel D, Gambarotti M, Alberghini M, Picci P, Faldini C. Errani C, et al. Skeletal Radiol. 2012 Dec;41(12):1495-507. doi: 10.1007/s00256-012-1510-6. Epub 2012 Sep 21. Skeletal Radiol. 2012. PMID: 22993209 Review. - Protective effect of low-intensity pulsed ultrasound on immune checkpoint inhibitor-related myocarditis via fine-tuning CD4+ T-cell differentiation.
Fu S, Guo Z, Xu X, Li Y, Choi S, Zhao P, Shen W, Gao F, Wang C, Chen S, Li Y, Tian J, Sun P. Fu S, et al. Cancer Immunol Immunother. 2024 Jan 18;73(1):15. doi: 10.1007/s00262-023-03590-5. Cancer Immunol Immunother. 2024. PMID: 38236243 Free PMC article. - YAP mediates the interaction between the Hippo and PI3K/Akt pathways in mesangial cell proliferation in diabetic nephropathy.
Qian X, He L, Hao M, Li Y, Li X, Liu Y, Jiang H, Xu L, Li C, Wu W, Du L, Yin X, Lu Q. Qian X, et al. Acta Diabetol. 2021 Jan;58(1):47-62. doi: 10.1007/s00592-020-01582-w. Epub 2020 Aug 20. Acta Diabetol. 2021. PMID: 32816106 - Regulation of TAZ in cancer.
Zhou X, Lei QY. Zhou X, et al. Protein Cell. 2016 Aug;7(8):548-61. doi: 10.1007/s13238-016-0288-z. Epub 2016 Jul 14. Protein Cell. 2016. PMID: 27412635 Free PMC article. Review.
References
- Hong, J. H., and Yaffe, M. B. (2006) Cell Cycle 5 176–179 - PubMed
- Hong, J. H., Hwang, E. S., McManus, M. T., Amsterdam, A., Tian, Y., Kalmukova, R., Mueller, E., Benjamin, T., Spiegelman, B. M., Sharp, P. A., Hopkins, N., and Yaffe, M. B. (2005) Science 309 1074–1078 - PubMed
- Murakami, M., Tominaga, J., Makita, R., Uchijima, Y., Kurihara, Y., Nakagawa, O., Asano, T., and Kurihara, H. (2006) Biochem. Biophys. Res. Commun. 339 533–539 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous