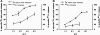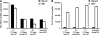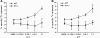Hydrolysis of tumor cell lipids after CTL-mediated death - PubMed (original) (raw)
Hydrolysis of tumor cell lipids after CTL-mediated death
Bryce Alves et al. Int Immunol. 2009 May.
Abstract
Contributions of lipases to CTL function have been debated, including if T cell lipases damage target cells. Expression of the lipase pancreatic lipase-related protein 2 (PLRP2) was previously found in IL-4 cultured lymphocyte cell lines but absent from IL-2 cultured lymphocytes. Here, we evaluated IL-2 and IL-4 induced CTLs for hydrolysis of target cell lipids and killing. Using anti-CD3 redirected lysis of [(3)H]-oleic acid-labeled P815 tumor cells, we detected the release of the radioactive fatty acid (FA). When PLRP2(+/+) and PLRP2(-/-) CTLs were compared, there was more killing by the PLRP2(+/+) CTLs. However, [(3)H]-oleic acid release was similar per dead P815, suggesting that lipid hydrolysis was produced by the dead P815s rather than by PLRP2. The FA release and death were completely dependent on perforin and also occurred when P815s were killed by perforin-containing T cell granule extracts that lacked lipase activity. Death by the cytotoxic granules extracts was unaffected by the addition of lipases. A lipase inhibitor, tetrahydrolipstatin, blocked FA release without affecting CTL-mediated cytotoxicity. Also, CTL-mediated death caused as much FA release as death by disruption of cells by freeze-thawing. The released oleic acid may be sufficient to promote secondary apoptotic responses after CTL-induced trauma.
Figures
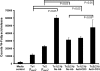
Fig. 1.
CTL release of triglyceride lipases. Splenocytes were activated and cultured with 500 U ml−1 of either murine r-IL-2 (to generate Tc1 CTLs) or r-IL-4 (to generate Tc2 CTLs). CTLs were incubated for 2.5 h with immobilized anti-CD3 to stimulate the T cell receptors for antigen and trigger exocytosis of cytotoxic granules and protein secretion. Each lipase assay contained 3.2 million cell equivalents. As a control for endogenous lipase activity present in the media and contributed by the washed CTLs, cell-free supernatants were taken at the Tzero points. The cell-free supernatants from the Tzero, Tc1 and Tc2 cell after stimulation, were concentrated and immediately assayed for lipase activity using radioactive triolein for 60 min at 37°C. Tc1 CTLs secreted a constitutive lipase activity that was decreased after anti-CD3 stimulation. In contrast, Tc2 cells secreted detectable lipase activity after anti-CD3 induced exocytosis. All _P_-values were determined using Student’s _t_-tests.
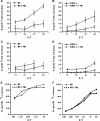
Fig. 2.
Release of [3H]-oleic acid from tumor cells after attack by cytotoxic T cells. P815 cells were incubated overnight with [3H]-oleic acid to label their lipids or labeled with 51Cr for 4 h to monitor cell death. The [3H]-radiolabel released into the cell-free supernatant was monitored as an initial indication of lipase activity (that would release oleic acid). Subsequently, we found that apoptotic bodies and membrane vesicles contributed to ∼50% of the [3H]-radiolabel in these supernatant (see Fig. 3). Release was proportional to the ratio of effector CTLs to P815 target cells (E:T). Tc1 or Tc2 CTLs, derived from BALB/c spleens and stimulated with either 500 U ml−1 IL-2 (A) or 500 U ml−1 IL-4 (B), respectively_,_ were used to redirect lysis of [3H]-oleic acid-labeled (closed triangles) or 51Cr-labeled P815 tumors (closed squares).
Fig. 3.
The cell-free supernatant contained soluble FAs after CTL attack. (A) Extractions of the cell-free supernatant indicated that roughly half of the released label was water-soluble oleic acid and that oleic acid-containing lipids were also elevated in the supernatants after cell death. (B) Extractions of cell pellets yielded nearly all oleic acid into the organic phase, where lipids partitioned. A loss in the cell-associated lipid counts was observed after redirected lysis.
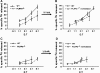
Fig. 4.
Release of the oleic acid-derived radiolabel was independent of PLRP2. CTLs derived from either PLRP2+/+ (solid line) or PLRP2−/− (dashed line) mice were cultured with 500 U ml−1 IL-2 (Tc1, A and B) or 500 U ml−1 IL-4 (Tc2, C and D) and used for redirected lysis of [3H]-oleic acid-labeled P815 cells (A–D). In the right column (B and D), the oleic acid release has been normalized to the death of the tumor cells, such that the graphs represent the amount of FA released relative to the number of cells killed. The arrows between the left and right columns indicate the correction factor used to normalize the oleic acid release to the number of dead cells. The correction factor was determined from the fold reduction in death exhibited by PLRP2−/− CTL when compared with PLRP2+/+ CTLs (Table 1). The WT CTLs with the PLRP2 gene were more lytic than the PLRP2−/− CTLs, particularly after culture with IL-4 to induce PLRP2 (3.5× more lytic). However, when the oleic acid release was normalized to cell death (see B and D), PLRP2 had insignificant effects on the amount of lipid radiolabel released during killing.
Fig. 5.
CTL release of FA was perforin dependent. CTLs were derived from WT and perforin knockout mice of the same C57BL/6 background, using conditions described for Fig. 2 and assayed for redirected lysis of [3H]-oleic acid-labeled or 51Cr-labeled P815 cells. There was substantial 51Cr lytic activity for the WT Tc1 and Tc2 CTLs that was totally absent from the Pfn−/− CTLs (data not illustrated). (A and B) In the absence of perforin, release of oleic acid was also undetectable for both Tc1 and Tc2 CTLs even at the highest ratio E:T ratio of 1:1.
Fig. 6.
Oleic acid release was sensitive to the lipase inhibitor THL, regardless of the WT or PLRP2−/− status of the CTLs. However, THL lacked effects on CTL-mediated cytotoxicity. CTLs were derived and tested as in Fig. 2. Inhibited assays received 10 μg ml−1 THL (dashed line), while control assays received DMSO as a control for the solvent added with THL (solid line). Both WT (A and C) and PLRP2−/− CTL release of oleic acid (B and D) were affected by THL. In (E and F), the P815s were labeled with 51Cr to monitor CTL-mediated lysis in the presence or absence of 10 μg ml−1 THL.
Similar articles
- Pancreatic lipase-related protein 2 (PLRP2) induction by IL-4 in cytotoxic T lymphocytes (CTLs) and reevaluation of the negative effects of its gene ablation on cytotoxicity.
Alves BN, Leong J, Tamang DL, Elliott V, Edelnant J, Redelman D, Singer CA, Kuhn AR, Miller R, Lowe ME, Hudig D. Alves BN, et al. J Leukoc Biol. 2009 Sep;86(3):701-12. doi: 10.1189/jlb.1208766. Epub 2009 May 18. J Leukoc Biol. 2009. PMID: 19451396 Free PMC article. - Lipid-dependent cytotoxicity by the lipase PLRP2 and by PLRP2-positive cytotoxic T lymphocytes (CTLs).
Alves BN, Marshall K, Tamang DL, Leong J, Redelman D, Elliott V, Lowe ME, Hudig D. Alves BN, et al. Cell Biochem Funct. 2009 Jul;27(5):296-308. doi: 10.1002/cbf.1573. Cell Biochem Funct. 2009. PMID: 19548271 Free PMC article. - Perforin-dependent and -independent pathways of cytotoxicity mediated by lymphocytes.
Young JD, Liu CC, Persechini PM, Cohn ZA. Young JD, et al. Immunol Rev. 1988 Mar;103:161-202. doi: 10.1111/j.1600-065x.1988.tb00755.x. Immunol Rev. 1988. PMID: 3292393 Review. - Pancreatic lipase and its related proteins: where are we now?
Yadav N, Paul AT. Yadav N, et al. Drug Discov Today. 2024 Jan;29(1):103855. doi: 10.1016/j.drudis.2023.103855. Epub 2023 Dec 9. Drug Discov Today. 2024. PMID: 38081381 Review.
Cited by
- HIF drives lipid deposition and cancer in ccRCC via repression of fatty acid metabolism.
Du W, Zhang L, Brett-Morris A, Aguila B, Kerner J, Hoppel CL, Puchowicz M, Serra D, Herrero L, Rini BI, Campbell S, Welford SM. Du W, et al. Nat Commun. 2017 Nov 24;8(1):1769. doi: 10.1038/s41467-017-01965-8. Nat Commun. 2017. PMID: 29176561 Free PMC article.
References
- Raja SM, Metkar SS, Froelich CJ. Cytotoxic granule-mediated apoptosis: unraveling the complex mechanism. Curr. Opin. Immunol. 2003;15:528. - PubMed
- Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449. - PubMed
- Koren HS, Ferber E, Fischer H. Changes in phospholipid metabolism of a tumor target cell during a cell-mediated cytotoxic reaction. Biochim. Biophys. Acta. 1971;231:520. - PubMed
- Iturralde M, Pardo J, Lacasa E, et al. Characterization of the lipolytic pathways that mediate free fatty acid release during Fas/CD95-induced apoptosis. Apoptosis. 2005;10:1369. - PubMed
- Grusby MJ, Nabavi N, Wong H, et al. Cloning of an interleukin-4 inducible gene from cytotoxic T lymphocytes and its identification as a lipase. Cell. 1990;60:451. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P20RR016463/RR/NCRR NIH HHS/United States
- T32 09563/PHS HHS/United States
- R01HD33060/HD/NICHD NIH HHS/United States
- R01CA38942-19/CA/NCI NIH HHS/United States
- DK053100/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases