Endogenous S-nitrosothiols protect against myocardial injury - PubMed (original) (raw)
. 2009 Apr 14;106(15):6297-302.
doi: 10.1073/pnas.0901043106. Epub 2009 Mar 26.
Gregory K W Lam, Liang Xie, Diana L Diesen, Nestor Villamizar, Jeffrey Nienaber, Emily Messina, Dawn Bowles, Christopher D Kontos, Joshua M Hare, Jonathan S Stamler, Howard A Rockman
Affiliations
- PMID: 19325130
- PMCID: PMC2669330
- DOI: 10.1073/pnas.0901043106
Endogenous S-nitrosothiols protect against myocardial injury
Brian Lima et al. Proc Natl Acad Sci U S A. 2009.
Abstract
Despite substantial evidence that nitric oxide (NO) and/or endogenous S-nitrosothiols (SNOs) exert protective effects in a variety of cardiovascular diseases, the molecular details are largely unknown. Here we show that following left coronary artery ligation, mice with a targeted deletion of the S-nitrosoglutathione reductase gene (GSNOR(-/-)) have reduced myocardial infarct size, preserved ventricular systolic and diastolic function, and maintained tissue oxygenation. These profound physiological effects are associated with increases in myocardial capillary density and S-nitrosylation of the transcription factor hypoxia inducible factor-1alpha (HIF-1alpha) under normoxic conditions. We further show that S-nitrosylated HIF-1alpha binds to the vascular endothelial growth factor (VEGF) gene, thus identifying a role for GSNO in angiogenesis and myocardial protection. These results suggest innovative approaches to modulate angiogenesis and preserve cardiac function.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
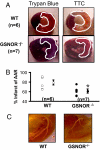
Fig. 1.
GSNOR−/− mice have smaller infarctions following coronary ligation. (A) Representative cross sections of wild type and GSNOR−/− hearts 48 h following LAD ligation. Before harvesting the heart from the euthanized mouse, the ascending aorta was cannulated to infuse trypan blue for demarcation of the area at risk (AAR, outlined in white). Following removal of the heart and sectioning, each myocardial section was photographed and then incubated in triphenyltetrazolium chloride (TTC) for demarcation of viable versus infarcted myocardium (outlined in white). (B) For each trypan blue myocardial segment, the corresponding TTC stained segment was photographed and used to calculate infarcted percentage of the AAR. The GSNOR−/− mice experienced an approximate 20% reduction in the infracted percentage of the AAR in comparison with the WT mice (*, P = 0.02, GSNOR−/− vs. WT). (C) Structural determination of coronary artery anatomy was performed using silicone casts, digestion of the myocardium, and digital photography. WT and GSNOR−/− mice exhibited similar coronary anatomy with a left main coronary artery that gives off a circumflex branch proximally and terminates in a bifurcation near the LV apex.
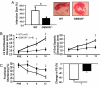
Fig. 2.
GSNOR−/− mice have less pathological remodeling and preserved left ventricular function 12 weeks after myocardial infarction. (A) At 12 wks following LAD ligation, mice were euthanized and hearts were harvested for infarction size determination. The GSNOR−/− mice displayed a markedly reduced infarction size compared to WT mice (17 ± 3% vs. 35 ± 10%, respectively; *, P = 0.03). Representative histological sections of infarcted LV myocardium in WT and GSNOR−/− mice demonstrate the decreased infarction size in a knockout heart. (B) WT and GSNOR−/− mice were evaluated with serial echocardiography immediately before, and for 12 wks following LAD ligation. Relative to the WT mice, which exhibited pathologic left ventricular remodeling with steady increase in LV end diastolic and end systolic dimensions (LV EDD, LV ESD), the GSNOR−/− mice experienced little increase in chamber dimensions after MI. (C) LV function, as determined by the percentage of fractional shortening and percent change in fractional shortening, declined to a significantly greater degree in the WT mice during the 12 wks following LAD ligation (*, P ≤ 0.03; †, P < 0.01; ‡, P < 0.001).
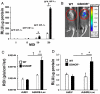
Fig. 3.
GSNOR−/− mice have increased baseline capillary density and preserved myocardial tissue oxygenation after ligation of the LAD artery. Myocardial immunohistochemical staining for capillary density using CD-31 antibody was performed in (A) WT and (B) GSNOR−/− mice. These representative sections show a higher level of positive CD-31 staining (white arrows) in the GSNOR−/− mouse heart, indicating increased capillary density. (C) Immunohistochemical analysis revealed a significantly greater number of CD-31 positive cells in the myocardium of GSNOR−/− mice (*, P = 0.002). (D) Myocardial partial pressure oxygen (pO2, mm Hg) measurements were recorded in WT and GSNOR−/− mice before and 15 min after ligation of the LAD artery (WT mice n = 10; GSNOR−/− mice, n = 8). Time-points represent averages of continuous readings taken over 10 seconds at each time interval. After 15 min of myocardial ischemia/infarct, WT mice experienced a significant decrease in myocardial pO2 compared to GSNOR−/− mice (†, P = 0.045).
Fig. 4.
GSNOR−/− mice exhibit enhanced HIF-1 transcriptional activity in normoxia. HeLa cells were transfected with either GFP or HIF-1α, 24 h before infection with Ad-HRE-luciferase at multiplicities of infection (MOIs) of 0, 10, 100, and 500. (A) The following day, the reporter assay was performed for luciferase activity, which demonstrated greatly increased luciferase activity with increased MOI of Ad-HRE-luciferase. Conversely, transfection with GFP did not induce an appreciable change in luciferase activity relative to HIF-1α (*, P ≤ 0.008; †, P < 0.001, HIF-1α vs. GFP). (B) On day 0, mice underwent single LV injection of empty adenovirus or Ad-HRE-luciferase, and, on day 3, they underwent in vivo bioluminescence evaluation. These representative images depict the significantly increased bioluminescence observed in the GSNOR−/− mice injected with Ad-HRE luciferase compared to WT mice (n = 3 per group). (C) In vivo quantification of emitted photons from these mice demonstrated a marked augmentation of luciferase activity in the GSNOR−/− mice injected with Ad-HRE-luciferase (†, P < 0.001, Ad-HRE-luciferase GSNOR−/− vs. WT; and GSNOR−/− Ad-HRE-luciferase vs. AdEV). (D) Ex vivo quantification of luciferase activity on myocardial tissue harvested on day 6 following viral injection revealed substantially increased relative light units (normalized for protein concentration) in the GSNOR−/− mice injected with Ad-HRE luciferase (*, P ≤ 0.008, Ad-HRE-luciferase GSNOR−/− vs. WT; and GSNOR−/− Ad-HRE-luciferase vs. AdEV).
Fig. 5.
HIF-1α protein stabilization and _S_-nitrosylation is increased in GSNOR−/− mice in normoxia. (A) Nuclear extracts from GSNOR−/− and WT myocardial tissue were analyzed for basal HIF-1α expression (n = 8) by western blot analysis. Normoxic HIF-1α expression was consistently higher in the GSNOR−/− mice compared to WT. (B) Densitometric analysis demonstrates a significant increase in the relative ratio of HIF-1α to histone in the GSNOR−/− mice compared to WT. The relative ratio of HIF-1α to histone in WT mice is arbitrarily presented as 1 (*, P = 0.03, relative density in GSNOR−/− mice 1.41 ± 0.28). (C) _S_-Nitrosylation of HIF-1α was determined in GSNOR−/− vs. WT mice by the biotin switch technique. _S_-nitrosylated HIF-1α (+ ascorbic acid, which enables labeling of SNO-protein with biotin) was consistently higher in the nuclear extracts of GSNOR−/− mice compared to WT in normoxic environments. (D) Densitometric analysis demonstrates a significant increase in the relative ratio of _S_-nitrosylated-HIF-1α to histone in the GSNOR−/− mice compared to WT. The relative ratio of _S_-nitrosylated HIF-1α to histone in WT mice is arbitrarily defined as 1 (†, P = 0.007, relative density in GSNOR−/− mice 1.62 ± 0.19). (E) Chromatin immunoprecipitation (ChIP) analysis of HIF-1α binding to the promoter region of the VEGF gene in W293 cells treated with increasing concentrations of GSNO. Immunoprecipitation of the HIF-1α-DNA complex revealed increases after treatment with GSNO.
Similar articles
- Protection from experimental asthma by an endogenous bronchodilator.
Que LG, Liu L, Yan Y, Whitehead GS, Gavett SH, Schwartz DA, Stamler JS. Que LG, et al. Science. 2005 Jun 10;308(5728):1618-21. doi: 10.1126/science.1108228. Epub 2005 May 26. Science. 2005. PMID: 15919956 Free PMC article. - Essential roles of S-nitrosothiols in vascular homeostasis and endotoxic shock.
Liu L, Yan Y, Zeng M, Zhang J, Hanes MA, Ahearn G, McMahon TJ, Dickfeld T, Marshall HE, Que LG, Stamler JS. Liu L, et al. Cell. 2004 Feb 20;116(4):617-28. doi: 10.1016/s0092-8674(04)00131-x. Cell. 2004. PMID: 14980227 - Reduction of cardiomyocyte S-nitrosylation by S-nitrosoglutathione reductase protects against sepsis-induced myocardial depression.
Sips PY, Irie T, Zou L, Shinozaki S, Sakai M, Shimizu N, Nguyen R, Stamler JS, Chao W, Kaneki M, Ichinose F. Sips PY, et al. Am J Physiol Heart Circ Physiol. 2013 Apr 15;304(8):H1134-46. doi: 10.1152/ajpheart.00887.2012. Epub 2013 Feb 15. Am J Physiol Heart Circ Physiol. 2013. PMID: 23417863 Free PMC article. - S-nitrosylation signaling in cell biology.
Gaston BM, Carver J, Doctor A, Palmer LA. Gaston BM, et al. Mol Interv. 2003 Aug;3(5):253-63. doi: 10.1124/mi.3.5.253. Mol Interv. 2003. PMID: 14993439 Review. - Novel s-nitrosothiols have potential therapeutic uses for cystic fibrosis.
Zaman K, Fraser-Butler M, Bennett D. Zaman K, et al. Curr Pharm Des. 2013;19(19):3509-20. doi: 10.2174/13816128113199990319. Curr Pharm Des. 2013. PMID: 23331028 Review.
Cited by
- Pharmacological inhibition of S-nitrosoglutathione reductase improves endothelial vasodilatory function in rats in vivo.
Chen Q, Sievers RE, Varga M, Kharait S, Haddad DJ, Patton AK, Delany CS, Mutka SC, Blonder JP, Dubé GP, Rosenthal GJ, Springer ML. Chen Q, et al. J Appl Physiol (1985). 2013 Mar 15;114(6):752-60. doi: 10.1152/japplphysiol.01302.2012. Epub 2013 Jan 24. J Appl Physiol (1985). 2013. PMID: 23349456 Free PMC article. - Thioredoxin-1 regulates cellular heme insertion by controlling S-nitrosation of glyceraldehyde-3-phosphate dehydrogenase.
Chakravarti R, Stuehr DJ. Chakravarti R, et al. J Biol Chem. 2012 May 11;287(20):16179-86. doi: 10.1074/jbc.M112.342758. Epub 2012 Mar 28. J Biol Chem. 2012. PMID: 22457359 Free PMC article. - Assessment of s-nitrosothiol and thiol/disulfide levels in acute coronary syndrome patients.
YiğitbaşI MM, Aslan AN, Kundi H, Erkılıç MF, Erel Ö, Kasapkara HA. YiğitbaşI MM, et al. Turk J Med Sci. 2022 Dec;52(6):1829-1838. doi: 10.55730/1300-0144.5529. Epub 2022 Dec 21. Turk J Med Sci. 2022. PMID: 36945993 Free PMC article. - Alcohol dehydrogenase 5 of Helicoverpa armigera interacts with the CYP6B6 promoter in response to 2-tridecanone.
Zhao J, Wei Q, Gu XR, Ren SW, Liu XN. Zhao J, et al. Insect Sci. 2020 Oct;27(5):1053-1066. doi: 10.1111/1744-7917.12720. Epub 2019 Sep 12. Insect Sci. 2020. PMID: 31454147 Free PMC article. - S-Nitrosoglutathione Reductase Is Essential for Protecting the Female Heart From Ischemia-Reperfusion Injury.
Casin KM, Fallica J, Mackowski N, Veenema RJ, Chan A, St Paul A, Zhu G, Bedja D, Biswal S, Kohr MJ. Casin KM, et al. Circ Res. 2018 Nov 9;123(11):1232-1243. doi: 10.1161/CIRCRESAHA.118.313956. Circ Res. 2018. PMID: 30571462 Free PMC article.
References
- Foster MW, McMahon TJ, Stamler JS. S-nitrosylation in health and disease. Trends Mol Med. 2003;9:160–168. - PubMed
- Gaston BM, Carver J, Doctor A, Palmer LA. S-nitrosylation signaling in cell biology. Mol Interv. 2003;3:253–263. - PubMed
- Liu L, et al. Essential roles of S-nitrosothiols in vascular homeostasis and endotoxic shock. Cell. 2004;116:617–628. - PubMed
- Whalen EJ, et al. Regulation of beta-adrenergic receptor signaling by S-nitrosylation of G-protein-coupled receptor kinase 2. Cell. 2007;129:511–522. - PubMed
- Hare JM. Nitric oxide and excitation-contraction coupling. J Mol Cell Cardiol. 2003;35:719–729. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases