Locating the functional and anatomical boundaries of human primary visual cortex - PubMed (original) (raw)
Locating the functional and anatomical boundaries of human primary visual cortex
Oliver Hinds et al. Neuroimage. 2009.
Abstract
The primary visual cortex (V1) can be delineated both functionally by its topographic map of the visual field and anatomically by its distinct pattern of laminar myelination. Although it is commonly assumed that the specialized anatomy V1 exhibits corresponds in location with functionally defined V1, demonstrating this in human has not been possible thus far due to the difficulty of determining the location of V1 both functionally and anatomically in the same individual. In this study we use MRI to measure the anatomical and functional V1 boundaries in the same individual and demonstrate close agreement between them. Functional V1 location was measured by parcellating occipital cortex of 10 living humans into visual cortical areas based on the topographic map of the visual field measured using functional MRI. Anatomical V1 location was estimated for these same subjects using a surface-based probabilistic atlas derived from high-resolution structural MRI of the stria of Gennari in 10 intact ex vivo human hemispheres. To ensure that the atlas prediction was correct, it was validated against V1 location measured using an observer-independent cortical parcellation based on the laminar pattern of cell density in serial brain sections from 10 separate individuals. The close agreement between the independent anatomically and functionally derived V1 boundaries indicates that the whole extent of V1 can be accurately predicted based on cortical surface reconstructions computed from structural MRI scans, eliminating the need for functional localizers of V1. In addition, that the primary cortical folds predict the location of functional V1 suggests that the mechanism giving rise to V1 location is tied to the development of the cortical folds.
Figures
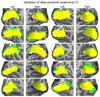
Figure 1
Validation of the surface-based probabilistic atlas of V1 on an independent dataset. An inflated representation of both the left and right hemispheres of each subject are shown. Surfaces were reconstructed from data used by Amunts et al. (2000) to study the variability of V1 in stereotaxic space. The location of anatomical V1 determined from histology is shown in blue, the location of V1 predicted using the probabilistic atlas of Hinds et al. (2008) thresholded at p = 0.8 is shown in green, and the region they share is colored in yellow. Close agreement between the atlas prediction and measured location is apparent.
Figure 2
Computing the atlas probability threshold that yields the minimum-bias estimate of the V1 boundary. (A) The mean signed boundary distance between the measured (from histology) and atlas-predicted V1 boundary, measured at atlas probabilities ranging from 0.1 to 1.0. The signed distance treats distances as negative if the predicted boundary is within the measured boundary and positive if outside. The minimum at 0.8 indicates that thresholding the probabilistic atlas at this level yields the minimum-bias V1 boundary prediction. (B) the mean unsigned boundary distance, which represents the more standard root-mean-square error between the boundary estimates.
Figure 3
V1 boundary comparison on the inflated cortex for an example subject. The location of anatomical V1 predicted by the probabilistic atlas shown in green. Locations determined to lie on the functional V1 boundary via fMRI are colored based on the measured surface-based distance to the nearest location on the anatomical V1 boundary.
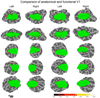
Figure 4
Alignment of the anatomical and functional V1 boundary. A portion of the flattened left and right occipital lobes is shown for each of the ten subjects with the location of anatomical V1 predicted by the probabilistic atlas shown in green. Locations determined to lie on the functional V1 boundary via fMRI are colored based on the measured surface-based distance to the nearest location on the anatomical V1 boundary. The color bar indicates the measured distance in mm. For each subject the occipital region of each hemisphere is shown with superior to the top and posterior to the left for left hemispheres and to the right for right hemispheres.
Figure 5
Agreement between independent measurements of V1 boundary location. (A) A histogram of the distance between the measured V1 boundary defined via analysis of histological data by Amunts et al. (2000) and the predicted V1 boundary based on the atlas of Hinds et al. (2008). The histogram represents the number of surface vertices on the atlas-predicted boundary that fall within a range of distances to the measured boundary. (B) A similar histogram where the distance computed is between each vertex of the functional boundary of V1 and the atlas-predicted boundary in the same subjects.
Similar articles
- Accurate prediction of V1 location from cortical folds in a surface coordinate system.
Hinds OP, Rajendran N, Polimeni JR, Augustinack JC, Wiggins G, Wald LL, Diana Rosas H, Potthast A, Schwartz EL, Fischl B. Hinds OP, et al. Neuroimage. 2008 Feb 15;39(4):1585-99. doi: 10.1016/j.neuroimage.2007.10.033. Epub 2007 Nov 6. Neuroimage. 2008. PMID: 18055222 Free PMC article. - Correspondence of human visual areas identified using functional and anatomical MRI in vivo at 7 T.
Sánchez-Panchuelo RM, Francis ST, Schluppeck D, Bowtell RW. Sánchez-Panchuelo RM, et al. J Magn Reson Imaging. 2012 Feb;35(2):287-99. doi: 10.1002/jmri.22822. Epub 2011 Sep 30. J Magn Reson Imaging. 2012. PMID: 21964755 - Linking retinotopic fMRI mapping and anatomical probability maps of human occipital areas V1 and V2.
Wohlschläger AM, Specht K, Lie C, Mohlberg H, Wohlschläger A, Bente K, Pietrzyk U, Stöcker T, Zilles K, Amunts K, Fink GR. Wohlschläger AM, et al. Neuroimage. 2005 May 15;26(1):73-82. doi: 10.1016/j.neuroimage.2005.01.021. Neuroimage. 2005. PMID: 15862207 Clinical Trial. - Borders, map clusters, and supra-areal organization in visual cortex.
Buckner RL, Yeo BT. Buckner RL, et al. Neuroimage. 2014 Jun;93 Pt 2(Pt 2):292-7. doi: 10.1016/j.neuroimage.2013.12.036. Epub 2013 Dec 27. Neuroimage. 2014. PMID: 24374078 Free PMC article. Review. - The chronoarchitecture of the cerebral cortex.
Bartels A, Zeki S. Bartels A, et al. Philos Trans R Soc Lond B Biol Sci. 2005 Apr 29;360(1456):733-50. doi: 10.1098/rstb.2005.1627. Philos Trans R Soc Lond B Biol Sci. 2005. PMID: 15937010 Free PMC article. Review.
Cited by
- Morphological patterns and spatial probability maps of the inferior frontal sulcus in the human brain.
Nolan E, Loh KK, Petrides M. Nolan E, et al. Hum Brain Mapp. 2024 Jul 15;45(10):e26759. doi: 10.1002/hbm.26759. Hum Brain Mapp. 2024. PMID: 38989632 Free PMC article. - The elusive metric of lesion load.
Seghier ML. Seghier ML. Brain Struct Funct. 2023 May;228(3-4):703-716. doi: 10.1007/s00429-023-02630-1. Epub 2023 Mar 22. Brain Struct Funct. 2023. PMID: 36947181 Review. - Homotopic local-global parcellation of the human cerebral cortex from resting-state functional connectivity.
Yan X, Kong R, Xue A, Yang Q, Orban C, An L, Holmes AJ, Qian X, Chen J, Zuo XN, Zhou JH, Fortier MV, Tan AP, Gluckman P, Chong YS, Meaney MJ, Bzdok D, Eickhoff SB, Yeo BTT. Yan X, et al. Neuroimage. 2023 Jun;273:120010. doi: 10.1016/j.neuroimage.2023.120010. Epub 2023 Mar 12. Neuroimage. 2023. PMID: 36918136 Free PMC article. - Functional ultrasound imaging reveals 3D structure of orientation domains in ferret primary visual cortex.
Hu W, Zhu S, Briggs F, Doyley MM. Hu W, et al. Neuroimage. 2023 Mar;268:119889. doi: 10.1016/j.neuroimage.2023.119889. Epub 2023 Jan 18. Neuroimage. 2023. PMID: 36681137 Free PMC article. - HOA2.0-ComPaRe: A next generation Harvard-Oxford Atlas comparative parcellation reasoning method for human and macaque individual brain parcellation and atlases of the cerebral cortex.
Rushmore RJ, Bouix S, Kubicki M, Rathi Y, Yeterian E, Makris N. Rushmore RJ, et al. Front Neuroanat. 2022 Nov 10;16:1035420. doi: 10.3389/fnana.2022.1035420. eCollection 2022. Front Neuroanat. 2022. PMID: 36439195 Free PMC article.
References
- Amunts K, Malikovic A, Mohlberg H, Schormann T, Zilles K. Brodmann’s areas 17 and 18 brought into stereotaxic space-where and how variable? Neuroimage. 2000;11(1):66–84. - PubMed
- Balasubramanian M, Polimeni JR, Schwartz EL. Exact geodesics and shortest paths on polyhedral surfaces. IEEE Trans Pattern Anal Mach Intell. 2009 In press. - PubMed
- Barbier E, Marrett S, Danek A, Vortmeyer A, van Gelderen P, Duyn J, Bandettini P, Grafman J, Koretsky AP. Imaging cortical anatomy by high-resolution MR at 3.0T: detection of the stripe of Gennari in visual area 17. Magn Reson Med. 2002;48(4):735–738. - PubMed
- Boyd J, Matsubara J. Repositioning the stria of Gennari [Abstract] Abstracts of the Society for Neuroscience. 2005
- Bridge H, Clare S, Jenkinson M, Jezzard P, Parker AJ, Matthews PM. Independent anatomical and functional measures of the V1/V2 boundary in human visual cortex. J Vis. 2005;5(2):93–102. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources