Remote astrocytic and microglial activation modulates neuronal hyperexcitability and below-level neuropathic pain after spinal injury in rat - PubMed (original) (raw)
Remote astrocytic and microglial activation modulates neuronal hyperexcitability and below-level neuropathic pain after spinal injury in rat
Y S Gwak et al. Neuroscience. 2009.
Abstract
In this study, we evaluated whether astrocytic and microglial activation mediates below-level neuropathic pain following spinal cord injury. Male Sprague-Dawley (225-250 g) rats were given low thoracic (T13) spinal transverse hemisection and behavioral, electrophysiological and immunohistochemical methods were used to examine the development and maintenance of below-level neuropathic pain. On postoperation day 28, both hind limbs showed significantly decreased paw withdrawal thresholds and thermal latencies as well as hyperexcitability of lumbar (L4-5) spinal wide dynamic range (WDR) neurons on both sides of spinal dorsal horn compared to sham controls (* P<0.05). Intrathecal treatment with propentofylline (PPF, 10 mM) for 7 consecutive days immediately after spinal injury attenuated the development of mechanical allodynia and thermal hyperalgesia in both hind limbs in a dose-related reduction compared to vehicle treatments (* P<0.05). Intrathecal treatment with single injections of PPF at 28 days after spinal injury, attenuated the existing mechanical allodynia and thermal hyperalgesia in both hind limbs in a dose related reduction (* P<0.05). In electrophysiological studies, topical treatment of 10 mM PPF onto the spinal surface attenuated the neuronal hyperexcitability in response to mechanical stimuli. In immunohistochemical studies, astrocytes and microglia in rats with spinal hemisection showed significantly increased GFAP and OX-42 expression in both superficial and deep dorsal horns in the lumbar spinal dorsal horn compared to sham controls (* P<0.05) that was prevented in a dose-related manner by PPF. In conclusion, our present data support astrocytic and microglial activation that contributes to below-level central neuropathic pain following spinal cord injury.
Figures
Figure 1
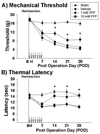
Partial prevention of mechanical allodynia and thermal hyperalgesia by early treatment with PPF. Early intrathecal treatment of 10 mM PPF (square, n=5) partially attenuated the development of mechanical allodynia (A) and thermal hyperalgesia (B) when compared to the vehicle treatment (diamond, n=5) whereas 1 mM PPF (circle, n=5) had no effect (*p<0.05). However, the sham group (triangle, n=3) did not display significant changes from presurgical values. Data are expressed as means ± S.E. Arrow : intrathecal injections of PPF.
Figure 2
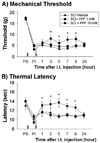
Attenuation of mechanical allodynia and thermal hyperalgesia by late intrathecal treatment of PPF on post operative day 28. Intrathecal (i.t.) treatment of 10 mM PPF (circle, n=5) partially attenuated the existing mechanical allodynia (A) and thermal hyperalgesia (B) when compared to the vehicle treatment (triangle, n=5, *p<0.05). However, 1 mM PPF (square, n=5) had no effect. Data are expressed as means ± S.E. Small arrow : hemisection, big arrow : intrathecal injection of PPF, PS : prior to hemisection, PI : prior to intrathecal injection (28 days after hemisection).
Figure 3
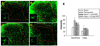
Morphological changes of spinal astrocytes in the lumbar dorsal horn following spinal transverse hemisection at T13. (A–D) Astrocytes visualized by GFAP immunoreaction product from spinal segments L4/L5 in the dorsal horn. (B) Post operation day (POD) 28 after hemisection (n=4), astrocytes in the entire dorsal horn displayed significantly increased area intensity (low magnification) and hypertrophy (high magnification) when compared to the sham control (A). (C) The intrathecal 1 mM PPF treatment did not display any significant changes when compared to hemisection groups. (D) The intrathecal 10 mM PPF treatment attenuated the increased area intensity and hypertrophy when compared to hemisection groups. (E) Intensity comparisons from immunoreaction products in both superficial and deep dorsal horns of sham controls (n=4), spinal hemisection (n=4), spinal hemisection + 1 mM PPF (Hemi + 1 mM PPF, n=4) and spinal hemisection + 10 mM PPF (Hemi + 10 mM PPF, n=4) for GFAP immunoreaction. Data are expressed as means ± S.E. *p<0.05-between sham and hemisection: #p<0.05-between hemisection and PPF treatments. Sampled images displayed in the whole dorsal horn (low magnification) and in representative cells from laminae IV–V (high magnification), respectively. Sacle bar : 300 µm.
Figure 4
Morphological changes of spinal microglia in the lumbar dorsal horn following spinal transverse hemisection at T13. (A–D) microglia visualized by OX-42 immunoreaction from spinal segments L4/L5 in the dorsal horn. (B) Post operation day (POD) 28 after hemisection, microglia in the entire dorsal horn displayed increased area intensity (low magnification) and hypertrophy (high magnification) when compared to the sham control (A). The intrathecal 1 mM PPF treatment (C) attenuated the increased area intensity and hypertrophy only in superficial dorsal horn whereas the intrathecal 10 mM PPF (D) treatment attenuated the increased area intensity and hypertrophy in both superficial and deep dorsal horns when compared to hemisection groups. (E) Intensity comparisons from immunoreaction products in both superficial and deep dorsal horns of sham controls, spinal hemisection, spinal hemisection + 1 mM PPF (Hemi + 1 mM PPF) and spinal hemisection + 10 mM PPF (Hemi + 10 mM PPF) for OX-42 immunoreaction. Data are expressed as means ± S.E. *p<0.05-between sham and hemisection: #p<0.05-between hemisection and PPF treatments. Sampled images displayed whole dorsal horn (low magnification) and representative cells from laminae IV–V (high magnification), respectively. Sacle bar : 300 µm.
Figure 5
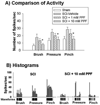
Attenuation of neuronal hyperexcitability by spinal treatment of PPF following spinal transverse hemisection at T13. (A) Spinal WDR dorsal horn neurons displayed significantly increased evoked activity in response to brush, pressure and pinch stimuli (10 seconds, each) after spinal hemisection (SCI-vehicle) compared to sham controls (n=5). Two hours after spinal treatment of 10 mM PPF (n=8), the activity was significantly attenuated whereas 1mM PPF (n=6) had no effect when compared to vehicle treatment groups (n=4, *p<0.05). (B) Real time peristimulus histograms with raw spike activity below in 10 sec bin widths (scale bar) during single neuron recordings from a spinal hemisected rat (SCI) and hemisection+10 mM PPF treatment rat (SCI + 10 mM PPF). Data are expressed as means ± S.E.
Similar articles
- Spatial and temporal activation of spinal glial cells: role of gliopathy in central neuropathic pain following spinal cord injury in rats.
Gwak YS, Kang J, Unabia GC, Hulsebosch CE. Gwak YS, et al. Exp Neurol. 2012 Apr;234(2):362-72. doi: 10.1016/j.expneurol.2011.10.010. Epub 2011 Oct 21. Exp Neurol. 2012. PMID: 22036747 Free PMC article. Review. - Activation of p-38alpha MAPK contributes to neuronal hyperexcitability in caudal regions remote from spinal cord injury.
Gwak YS, Unabia GC, Hulsebosch CE. Gwak YS, et al. Exp Neurol. 2009 Nov;220(1):154-61. doi: 10.1016/j.expneurol.2009.08.012. Epub 2009 Aug 20. Exp Neurol. 2009. PMID: 19699199 Free PMC article. - Activation of astrocytes in the spinal cord contributes to the development of bilateral allodynia after peripheral nerve injury in rats.
Obata H, Sakurazawa S, Kimura M, Saito S. Obata H, et al. Brain Res. 2010 Dec 2;1363:72-80. doi: 10.1016/j.brainres.2010.09.105. Epub 2010 Oct 25. Brain Res. 2010. PMID: 20932955 - Propentofylline attenuates allodynia, glial activation and modulates GABAergic tone after spinal cord injury in the rat.
Gwak YS, Crown ED, Unabia GC, Hulsebosch CE. Gwak YS, et al. Pain. 2008 Aug 31;138(2):410-422. doi: 10.1016/j.pain.2008.01.021. Epub 2008 Mar 18. Pain. 2008. PMID: 18353556 Free PMC article. - Neuronal hyperexcitability: a substrate for central neuropathic pain after spinal cord injury.
Gwak YS, Hulsebosch CE. Gwak YS, et al. Curr Pain Headache Rep. 2011 Jun;15(3):215-22. doi: 10.1007/s11916-011-0186-2. Curr Pain Headache Rep. 2011. PMID: 21387163 Review.
Cited by
- Chronic spontaneous activity generated in the somata of primary nociceptors is associated with pain-related behavior after spinal cord injury.
Bedi SS, Yang Q, Crook RJ, Du J, Wu Z, Fishman HM, Grill RJ, Carlton SM, Walters ET. Bedi SS, et al. J Neurosci. 2010 Nov 3;30(44):14870-82. doi: 10.1523/JNEUROSCI.2428-10.2010. J Neurosci. 2010. PMID: 21048146 Free PMC article. - Spatial and temporal activation of spinal glial cells: role of gliopathy in central neuropathic pain following spinal cord injury in rats.
Gwak YS, Kang J, Unabia GC, Hulsebosch CE. Gwak YS, et al. Exp Neurol. 2012 Apr;234(2):362-72. doi: 10.1016/j.expneurol.2011.10.010. Epub 2011 Oct 21. Exp Neurol. 2012. PMID: 22036747 Free PMC article. Review. - Emerging role of astroglia in pain hypersensitivity.
Ren K. Ren K. Jpn Dent Sci Rev. 2010 Feb 1;46(1):86. doi: 10.1016/j.jdsr.2009.10.005. Jpn Dent Sci Rev. 2010. PMID: 20161683 Free PMC article. - Purinergic Modulation of Spinal Neuroglial Maladaptive Plasticity Following Peripheral Nerve Injury.
Cirillo G, Colangelo AM, Berbenni M, Ippolito VM, De Luca C, Verdesca F, Savarese L, Alberghina L, Maggio N, Papa M. Cirillo G, et al. Mol Neurobiol. 2015 Dec;52(3):1440-1457. doi: 10.1007/s12035-014-8943-y. Epub 2014 Oct 29. Mol Neurobiol. 2015. PMID: 25352445 - Neural progenitor cells but not astrocytes respond distally to thoracic spinal cord injury in rat models.
Nguyen T, Mao Y, Sutherland T, Gorrie CA. Nguyen T, et al. Neural Regen Res. 2017 Nov;12(11):1885-1894. doi: 10.4103/1673-5374.219051. Neural Regen Res. 2017. PMID: 29239336 Free PMC article.
References
- Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32:1–14. - PubMed
- Basso M, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. - PubMed
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. - PubMed
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Meth. 1994;53:55–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 NS039161/NS/NINDS NIH HHS/United States
- P01 NS039161-05/NS/NINDS NIH HHS/United States
- P01 NS011255-27A19007/NS/NINDS NIH HHS/United States
- NS11255/NS/NINDS NIH HHS/United States
- P01 NS011255/NS/NINDS NIH HHS/United States
- P01 NS011255-32A20040/NS/NINDS NIH HHS/United States
- P01 NS011255-310037/NS/NINDS NIH HHS/United States
- P01 NS039161-01A2/NS/NINDS NIH HHS/United States
- NS39161/NS/NINDS NIH HHS/United States
- P01 NS011255-32A29010/NS/NINDS NIH HHS/United States
- P01 NS011255-230037/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous