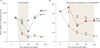VEGF inhibitors in the treatment of cerebral edema in patients with brain cancer - PubMed (original) (raw)
Review
VEGF inhibitors in the treatment of cerebral edema in patients with brain cancer
Elizabeth R Gerstner et al. Nat Rev Clin Oncol. 2009 Apr.
Abstract
Most brain tumors oversecrete vascular endothelial growth factor (VEGF), which leads to an abnormally permeable tumor vasculature. This hyperpermeability allows fluid to leak from the intravascular space into the brain parenchyma, which causes vasogenic cerebral edema and increased interstitial fluid pressure. Increased interstitial fluid pressure has an important role in treatment resistance by contributing to tumor hypoxia and preventing adequate tumor penetration of chemotherapy agents. In addition, edema and the corticosteroids needed to control cerebral edema cause significant morbidity and mortality. Agents that block the VEGF pathway are able to decrease vascular permeability and, thus, cerebral edema, by restoring the abnormal tumor vasculature to a more normal state. Decreasing cerebral edema minimizes the adverse effects of corticosteroids and could improve clinical outcomes. Anti-VEGF agents might also be useful in other cancer-related conditions that increase vascular permeability, such as malignant pleural effusions or ascites.
Figures
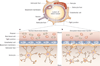
Figure 1
The permeability of junctions in normal and disrupted blood–brain barrier. a | The normal blood–brain barrier is composed of an intricate network of astrocytes, pericytes, endothelial cells, and neurons that form tight, impermeable junctions, which exclude large cells, marcomolecules, and excess fluid from the central nervous system. b | In the setting of a brain tumor, the tumor astrocytes are more densely packed and irregular, the basement membrane is disrupted and thickened, and the tight junctions are widened, allowing passage of macromolecules and fluid.
Figure 2
Schematic diagram to illustrate the process of vascular normalization a | In health, an exquisite balance of signaling from proangiogenic and antiangiogenic molecules maintains an organized and efficient vascular supply. b | Tumors produce angiogenic factors (various shades of green) that induce an abnormal, inefficient vascular network. c | Judiciously administered antiangiogenic therapy can bring the balance back to a more normal state and normalize the vascular network, improving drug delivery and efficacy. d | If antiangiogenesis is potent and/or persistent it can destroy the network totally, impeding delivery of oxygen and nutrients, and ultimately starving the tumor. In preclinical models, panel c usually progresses towards panel d (single arrow) with currently approved antiangiogenic agents. However, in human tumors, panel c commonly reverts to panel b after a ‘window of normalization’ (double arrow). Abbreviations: Anti, antiangiogenic molecules; IFP, interstitial fluid pressure; pO2, tissue oxygen level; Pro, proangiogenic molecules.
Figure 3
Effects of cediranib on cerebral edema in patients with recurrent glioblastoma. a | Median values for contrast-enhanced T1-weighted tumor volume (CE-T1), vessel size (VS), and permeability (P) of the tumor over time as measured by an independent expert. Day –1 was set as 100% in all tumors, and changes during cediranib treatment were plotted for all patients. Note the rebound of CE-T1 volume and vessel size after day 28, which indicates a partial closure of the normalization window. By contrast, permeability (P) remains diminished for a longer period of time. b | Median values of potential MRI markers of cerebral edema including T2-weighted abnormality volume measured by fluid attenuated inversion recovery images (FLAIR), apparent diffusion coefficient (ADC), and extracellular–extravascular volume fraction (v_e), before and during treatment, showing a sustained decrease of edema while taking cediranib. a_P <0.05 for values compared with day –1. b_P_ <0.05 for values compared with day +1. Abbreviations: ADC, apparent diffusion coefficient; CE-T1, contrast-enhanced T1-weighted tumor volume; FLAIR, fluid attenuation inversion recovery; P, permeability; _v_e, extracellular–extravascular volume fraction; VS, vessel size. Permission obtained from Elsevier © Batchelor, T. T. et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell 11, 83–95 (2007).
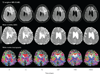
Figure 4
serial MRI images from the same patient (as shown in Figure 3) with a left frontal glioblastoma treated with cediranib. Days –5 and –1 are baseline studies before initiation of treatment, followed by images on day +1, +27, +55, +111 after cediranib therapy. Top row: FLAIR images demonstrating resolution of edema with treatment. Second row: Apparent diffusion coefficient maps (measurement of water mobility) demonstrating improved water mobility (that is, less edema causing restricted diffusion) with treatment. Bottom row: White matter tractography demonstrating improvement in the architecture of white matter fiber tracts as edema resolves and mass effect and tissue displacement diminish. Abbreviations: ADC, apparent diffusion coefficient; FLAIR, fluid attenuation inversion recovery. Permission obtained from Elsevier © Batchelor, T. T., et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell 11, 83–95 (2007).
Similar articles
- The genesis of peritumoral vasogenic brain edema and tumor cysts: a hypothetical role for tumor-derived vascular permeability factor.
Criscuolo GR. Criscuolo GR. Yale J Biol Med. 1993 Jul-Aug;66(4):277-314. Yale J Biol Med. 1993. PMID: 7516104 Free PMC article. Review. - AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients.
Batchelor TT, Sorensen AG, di Tomaso E, Zhang WT, Duda DG, Cohen KS, Kozak KR, Cahill DP, Chen PJ, Zhu M, Ancukiewicz M, Mrugala MM, Plotkin S, Drappatz J, Louis DN, Ivy P, Scadden DT, Benner T, Loeffler JS, Wen PY, Jain RK. Batchelor TT, et al. Cancer Cell. 2007 Jan;11(1):83-95. doi: 10.1016/j.ccr.2006.11.021. Cancer Cell. 2007. PMID: 17222792 Free PMC article. Clinical Trial. - Vascular permeability factor in brain metastases: correlation with vasogenic brain edema and tumor angiogenesis.
Strugar J, Rothbart D, Harrington W, Criscuolo GR. Strugar J, et al. J Neurosurg. 1994 Oct;81(4):560-6. doi: 10.3171/jns.1994.81.4.0560. J Neurosurg. 1994. PMID: 7523634 - Angiogenesis in brain tumours.
Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT. Jain RK, et al. Nat Rev Neurosci. 2007 Aug;8(8):610-22. doi: 10.1038/nrn2175. Nat Rev Neurosci. 2007. PMID: 17643088 Review. - Edema control by cediranib, a vascular endothelial growth factor receptor-targeted kinase inhibitor, prolongs survival despite persistent brain tumor growth in mice.
Kamoun WS, Ley CD, Farrar CT, Duyverman AM, Lahdenranta J, Lacorre DA, Batchelor TT, di Tomaso E, Duda DG, Munn LL, Fukumura D, Sorensen AG, Jain RK. Kamoun WS, et al. J Clin Oncol. 2009 May 20;27(15):2542-52. doi: 10.1200/JCO.2008.19.9356. Epub 2009 Mar 30. J Clin Oncol. 2009. PMID: 19332720 Free PMC article.
Cited by
- Targeting TRAF3IP2 inhibits angiogenesis in glioblastoma.
Izadpanah A, Daneshimehr F, Willingham K, Barabadi Z, Braun SE, Dumont A, Mostany R, Chandrasekar B, Alt EU, Izadpanah R. Izadpanah A, et al. Front Oncol. 2022 Aug 15;12:893820. doi: 10.3389/fonc.2022.893820. eCollection 2022. Front Oncol. 2022. PMID: 36046049 Free PMC article. - Prediction of glioblastoma multiform response to bevacizumab treatment using multi-parametric MRI.
Najafi M, Soltanian-Zadeh H, Jafari-Khouzani K, Scarpace L, Mikkelsen T. Najafi M, et al. PLoS One. 2012;7(1):e29945. doi: 10.1371/journal.pone.0029945. Epub 2012 Jan 11. PLoS One. 2012. PMID: 22253835 Free PMC article. - Regulation of Tumor Invasion by the Physical Microenvironment: Lessons from Breast and Brain Cancer.
Beeghly GF, Amofa KY, Fischbach C, Kumar S. Beeghly GF, et al. Annu Rev Biomed Eng. 2022 Jun 6;24:29-59. doi: 10.1146/annurev-bioeng-110220-115419. Epub 2022 Feb 4. Annu Rev Biomed Eng. 2022. PMID: 35119915 Free PMC article. Review. - Neuro-oncologic Emergencies.
Suarez-Meade P, Marenco-Hillembrand L, Sherman WJ. Suarez-Meade P, et al. Curr Oncol Rep. 2022 Aug;24(8):975-984. doi: 10.1007/s11912-022-01259-3. Epub 2022 Mar 30. Curr Oncol Rep. 2022. PMID: 35353348 Review. - Assessment of Glioblastoma Response in the Era of Bevacizumab: Longstanding and Emergent Challenges in the Imaging Evaluation of Pseudoresponse.
Arevalo OD, Soto C, Rabiei P, Kamali A, Ballester LY, Esquenazi Y, Zhu JJ, Riascos RF. Arevalo OD, et al. Front Neurol. 2019 May 7;10:460. doi: 10.3389/fneur.2019.00460. eCollection 2019. Front Neurol. 2019. PMID: 31133966 Free PMC article. Review.
References
- Del Maestro RF, Megyesi JF, Farrell CL. Mechanisms of tumour-associated edema: a review. Can. J. Neurol. Sci. 1990;17:177–183. - PubMed
- Klatzo I. Presidential address. Neuropathological aspects of brain edema. J. Neuropathol. Exp. Neurol. 1967;26:1–14. - PubMed
- Eichler AF, Loeffler JS. Multidisciplinary management of brain metastases. Oncologist. 2007;12:884–898. - PubMed
- Carlson M, et al. Relationship between survival and edema in malignant gliomas: role of vascular endothelial growth factor and neuronal pentraxin 2. Clin. Cancer Res. 2007;13:2592–2598. - PubMed
- Kofman S, Garvin JS, Nagamani D, Taylor SG., 3rd Treatment of cerebral metastases from breast carcinoma with prednisolone. J. Am. Med. Assoc. 1957;163:1473–1476. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources