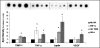Role of hyaluronan in angiogenesis and its utility to angiogenic tissue engineering - PubMed (original) (raw)
Role of hyaluronan in angiogenesis and its utility to angiogenic tissue engineering
Erin L Pardue et al. Organogenesis. 2008 Oct.
Abstract
Angiogenesis represents the outgrowth of new blood vessels from existing ones, a physiologic process that is vital to supply nourishment to newly forming tissues during development and tissue remodeling and repair (wound healing). Regulation of angiogenesis in the healthy body occurs through a fine balance of angiogenesis-stimulating factors and angiogenesis inhibitors. When this balance is disturbed, excessive or deficient angiogenesis can result and contribute to development of a wide variety of pathological conditions. The therapeutic stimulation or suppression of angiogenesis could be the key to abrogating these diseases. In recent years, tissue engineering has emerged as a promising technology for regenerating tissues or organs that are diseased beyond repair. Among the critical challenges that deter the practical realization of the vision of regenerating functional tissues for clinical implantation, is how tissues of finite size can be regenerated and maintained viable in the long-term. Since the diffusion of nutrients and essential gases to cells, and removal of metabolic wastes is typically limited to a depth of 150-250 microm from a capillary (3-10 cells thick), tissue constructs must mandatorily permit in-growth of a blood capillary network to nourish and sustain the viability of cells within. The purpose of this article is to provide an overview of the role and significance of hyaluronan (HA), a glycosaminoglycan (GAG) component of connective tissues, in physiologic and pathological angiogenesis, its applicability as a therapeutic to stimulate or suppress angiogenesis in situ within necrotic tissues in vivo, and the factors determining its potential utility as a pro-angiogenic stimulus that will enable tissue engineering of neo-vascularized and functional tissue constructs for clinical use.
Keywords: angiogenesis; hyaluronan; neo-vascularization; oligosaccharides; regenerative medicine; tissue engineering.
Figures
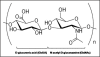
Figure 1
The chemical structure of HA. The HA polymer may contain up to 25,000 repeats of the disaccharide units of N-acetyl-D-glucosamine (GlcNAc) and D-glucuronic acid (GlcUA) linked together by alternating 1 → 3 and 1 → 4 glycosidic bonds. Each repeating disaccharide unit has one carboxylate group, four hydroxyl groups and an acetamido group. The negative charge of the molecule is due to ionization of the carboxyl groups of the glucuronic acid constituents at physiological pH.
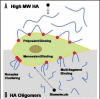
Figure 2
MW-dependant interactions of HA with cells. (A) High MW HA binds cells and extracellular hyaladherins in a polyvalent manner to protect and maintain the structure and integrity of tissues with few stimulatory effects on cells. The HA also tends to form pericellular sheaths that prevent cell-cell and cell-growth factor interactions. (B) In contrast, HA oligomers interact with cellular receptors in a monovalent manner, and may cause clustering of cell surface receptors (e.g., CD44) to evoke a multitude of intracellular signaling cascades.
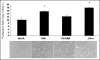
Figure 3
Proliferation of EC cultures in response to exogenous HA supplements (2 micrograms/ml). The proliferation ratios of ECs treated with pure HA 6mers and digests (HA-o) containing HA 6mers (33.3 ± 2.4% w/w) and 12mers (39.2 ± 2.7% w/w) proliferated to a greater degree than cells cultured with HA of MW = 1500 KDa or no HA. Morphology of ECs cultured with HA supplements remained similar to HA-free control cells. *denotes a p-value < 0.05 in comparison to the no HA control. Results adapted from reference .
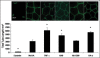
Figure 4
Impact of HA and HA oligomers on EC tube formation on matrigel. HA of MW = 1500 kDa increased individual tube lengths but decreased tube densities resulting in a cumulative tube length similar to the non-HA control cultures. Lengths of individual tubes in TNF-α HA 6mer- and HA-o- (see composition in Figure 3) stimulated cultures were similar to that in the controls but the number of tubes were significantly greater, resulting in a greater cumulative tube length. Suramin drastically inhibited tube formation and tubes in TNFα-supplemented cultures seemed to be incomplete, with gaps between junctions. Tube formation images were labeled with calcein AM *denotes a p-value < 0.05 in comparison to the no HA control. All HA supplements were added at a dose of 2 µg/ml. Results adapted from.
Figure 5
Impact of HA and HA oligomers on production of angiogenic cytokines by cultured rat aortic ECs. Of the 19 cytokines tested in each cytokine array, here we report three that are specifically known to influence angiogenesis. HA-oligomers (HA-o) upregulated production of pro-angiogenic TNFα, Leptin, and VEGF but also enhanced production of tissue inhibitor of matrix metalloproteinases (TIMP-1), an inhibitor of angiogenesis in vivo. HMW HA (HA 1500) had no effect on VEGF, Leptin, and TNFα production, but enhanced TIMP-1 synthesis, suggesting anti-angiogenic effects. HA dose was 2 micrograms/ml. *denotes a p-value < 0.05 in comparison to the no HA control. Results adapted from reference .
Similar articles
- Engineering blood vessels and vascularized tissues: technology trends and potential clinical applications.
Chandra P, Atala A. Chandra P, et al. Clin Sci (Lond). 2019 May 14;133(9):1115-1135. doi: 10.1042/CS20180155. Print 2019 May 15. Clin Sci (Lond). 2019. PMID: 31088895 Review. - [Implication of hyaluronic acid in normal and pathological angiogenesis. Application for cellular engineering].
Lataillade JJ, Albanese P, Uzan G. Lataillade JJ, et al. Ann Dermatol Venereol. 2010 Apr;137 Suppl 1:S15-22. doi: 10.1016/S0151-9638(10)70004-1. Ann Dermatol Venereol. 2010. PMID: 20435250 Review. French. - Injectable and tunable hyaluronic acid hydrogels releasing chemotactic and angiogenic growth factors for endodontic regeneration.
Silva CR, Babo PS, Gulino M, Costa L, Oliveira JM, Silva-Correia J, Domingues RMA, Reis RL, Gomes ME. Silva CR, et al. Acta Biomater. 2018 Sep 1;77:155-171. doi: 10.1016/j.actbio.2018.07.035. Epub 2018 Jul 18. Acta Biomater. 2018. PMID: 30031163 - Hyaluronan-mediated angiogenesis in vascular disease: uncovering RHAMM and CD44 receptor signaling pathways.
Slevin M, Krupinski J, Gaffney J, Matou S, West D, Delisser H, Savani RC, Kumar S. Slevin M, et al. Matrix Biol. 2007 Jan;26(1):58-68. doi: 10.1016/j.matbio.2006.08.261. Epub 2006 Sep 19. Matrix Biol. 2007. PMID: 17055233 Review. - Bioprinting for vascular and vascularized tissue biofabrication.
Datta P, Ayan B, Ozbolat IT. Datta P, et al. Acta Biomater. 2017 Mar 15;51:1-20. doi: 10.1016/j.actbio.2017.01.035. Epub 2017 Jan 11. Acta Biomater. 2017. PMID: 28087487 Review.
Cited by
- Composition and Anticoagulant Potential of Chondroitin Sulfate and Dermatan Sulfate from Inedible Parts of Garfish (Belone belone).
Chikha SB, Bougatef H, Capitani F, Ben Amor I, Maccari F, Gargouri J, Sila A, Volpi N, Bougatef A. Chikha SB, et al. Foods. 2023 Oct 24;12(21):3887. doi: 10.3390/foods12213887. Foods. 2023. PMID: 37959006 Free PMC article. - Hyaluronic acid facilitates bone repair effects of calcium phosphate cement by accelerating osteogenic expression.
Cui X, Huang C, Chen Z, Zhang M, Liu C, Su K, Wang J, Li L, Wang R, Li B, Chen D, Ruan C, Wang D, Lu WW, Pan H. Cui X, et al. Bioact Mater. 2021 Apr 8;6(11):3801-3811. doi: 10.1016/j.bioactmat.2021.03.028. eCollection 2021 Nov. Bioact Mater. 2021. PMID: 33937587 Free PMC article. - Coating Solid Lipid Nanoparticles with Hyaluronic Acid Enhances Antitumor Activity against Melanoma Stem-like Cells.
Shen H, Shi S, Zhang Z, Gong T, Sun X. Shen H, et al. Theranostics. 2015 Apr 5;5(7):755-71. doi: 10.7150/thno.10804. eCollection 2015. Theranostics. 2015. PMID: 25897340 Free PMC article. - Injectable Supramolecular Hydrogel/Microgel Composites for Therapeutic Delivery.
Chen MH, Chung JJ, Mealy JE, Zaman S, Li EC, Arisi MF, Atluri P, Burdick JA. Chen MH, et al. Macromol Biosci. 2019 Jan;19(1):e1800248. doi: 10.1002/mabi.201800248. Epub 2018 Sep 27. Macromol Biosci. 2019. PMID: 30259658 Free PMC article. - Glycocalyx-Sodium Interaction in Vascular Endothelium.
Sembajwe LF, Ssekandi AM, Namaganda A, Muwonge H, Kasolo JN, Kalyesubula R, Nakimuli A, Naome M, Patel KP, Masenga SK, Kirabo A. Sembajwe LF, et al. Nutrients. 2023 Jun 25;15(13):2873. doi: 10.3390/nu15132873. Nutrients. 2023. PMID: 37447199 Free PMC article. Review.
References
- Folkman J. Toward an understanding of angiogenesis: search and discovery. Perspect Biol Med. 1985;29:10–36. - PubMed
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. - PubMed
- Laschke MW, Harder Y, Amon M, et al. Angiogenesis in tissue engineering: breathing life into constructed tissue substitutes. Tissue Eng. 2006;12:2093–2104. - PubMed
- Arnold F, West DC. Angiogenesis in wound healing. Pharmacol Ther. 1991;52:407–422. - PubMed
- Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA. Integrin alphavbeta3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994;79:1157–1164. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources