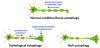The cellular pathways of neuronal autophagy and their implication in neurodegenerative diseases - PubMed (original) (raw)
Review
The cellular pathways of neuronal autophagy and their implication in neurodegenerative diseases
Zhenyu Yue et al. Biochim Biophys Acta. 2009 Sep.
Abstract
Autophagy is a tightly regulated cell self-eating process. It has been shown to be associated with various neuropathological conditions and therefore, traditionally known as a stress-induced process. Recent studies, however, reveal that autophagy is constitutively active in healthy neurons. Neurons are highly specialized, post-mitotic cells that are typically composed of a soma (cell body), a dendritic tree, and an axon. Despite the vast growth of our current knowledge of autophagy, the detailed process in such a highly differentiated cell type remains elusive. Current evidence strongly suggests that autophagy is uniquely regulated in neurons and is also highly adapted to local physiology in the axons. In addition, the molecular mechanism for basal autophagy in neurons may be significantly divergent from "classical" induced autophagy. A considerable number of studies have increasingly shown an important role for autophagy in neurodegenerative diseases and have explored autophagy as a potential drug target. Thus, understanding the neuronal autophagy process will ultimately aid in drug target identification and rational design of drug screening to combat neurodegenerative diseases.
Figures
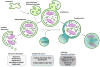
Figure 1
A schematic representation of the macroautophagy process. Following induction, a phagophore or isolation membrane is formed. This process is regulated by Atg1 (Ulk1), Atg9, and the PtdIns 3-kinase complex, which includes Beclin 1, Atg14L, and UVRAG (nucleation). Two ubiquitin-like conjugation systems, which produce Atg8-PE (LC3II) and Atg5-Atg12, mediate the elongation of the isolation membrane, closure, and the formation a double-membrane vacuole known as the autophagosome. Autophagosomes can undergo maturation and fusion with early and late endosomes and MVBs, to generate the amphisome, followed by fusion with lysosomes, to form the autolysosome. Trafficking, maturation, and fusion events are mediated by microtubules and specific SNARE and Rab proteins. ESCRT proteins are also essential for MVB fusion with autophagosomes. Alternatively, immature autophagosomes can fuse directly with lysosomes.
Figure 2
Proposed model for neuronal autophagy in the axons. Under normal conditions, basal autophagy maintains axonal homeostasis by removal of “autophagic cargo” (top). The “autophagic cargo” undergoes retrograde axonal transport to the soma and fuses with lysosomes for degradation. Stress or injury can induce local biosynthesis of autophagosomes, resulting in their accumulation in axons and axon terminals. It has been suggested that, under pathological conditions, precursors of degradative vesicles or lysosomes may be anterogradely transported to the axon terminal and contribute to the degradation of autophagosomes (bottom left). Neurons deficient in autophagy amass proteins, organelles, and aberrant membrane structures at axon terminals, resulting in gross axonal swellings or dystrophy (bottom right).
Similar articles
- Neuronal autophagy: going the distance to the axon.
Yue Z, Wang QJ, Komatsu M. Yue Z, et al. Autophagy. 2008 Jan;4(1):94-6. doi: 10.4161/auto.5202. Epub 2007 Oct 23. Autophagy. 2008. PMID: 18000396 Free PMC article. - Compartment-Specific Regulation of Autophagy in Primary Neurons.
Maday S, Holzbaur EL. Maday S, et al. J Neurosci. 2016 Jun 1;36(22):5933-45. doi: 10.1523/JNEUROSCI.4401-15.2016. J Neurosci. 2016. PMID: 27251616 Free PMC article. - Autophagosome dynamics in neurodegeneration at a glance.
Wong YC, Holzbaur EL. Wong YC, et al. J Cell Sci. 2015 Apr 1;128(7):1259-67. doi: 10.1242/jcs.161216. J Cell Sci. 2015. PMID: 25829512 Free PMC article. Review. - [Neuronal autophagy, its defects and neurodegenerative diseases].
Komatsu M, Ichimura Y. Komatsu M, et al. Tanpakushitsu Kakusan Koso. 2008 Dec;53(16 Suppl):2175-81. Tanpakushitsu Kakusan Koso. 2008. PMID: 21038604 Review. Japanese. No abstract available. - Autophagy in neurons: a review.
Larsen KE, Sulzer D. Larsen KE, et al. Histol Histopathol. 2002;17(3):897-908. doi: 10.14670/HH-17.897. Histol Histopathol. 2002. PMID: 12168801 Review.
Cited by
- Control of autophagy as a therapy for neurodegenerative disease.
Harris H, Rubinsztein DC. Harris H, et al. Nat Rev Neurol. 2011 Dec 20;8(2):108-17. doi: 10.1038/nrneurol.2011.200. Nat Rev Neurol. 2011. PMID: 22187000 Review. - FTY720 (Fingolimod) Ameliorates Brain Injury through Multiple Mechanisms and is a Strong Candidate for Stroke Treatment.
Wang Z, Kawabori M, Houkin K. Wang Z, et al. Curr Med Chem. 2020;27(18):2979-2993. doi: 10.2174/0929867326666190308133732. Curr Med Chem. 2020. PMID: 31785606 Free PMC article. Review. - The elimination of accumulated and aggregated proteins: a role for aggrephagy in neurodegeneration.
Yamamoto A, Simonsen A. Yamamoto A, et al. Neurobiol Dis. 2011 Jul;43(1):17-28. doi: 10.1016/j.nbd.2010.08.015. Epub 2010 Aug 20. Neurobiol Dis. 2011. PMID: 20732422 Free PMC article. Review. - Autophagic processes increase during senescence in cultured sheep neurons and astrocytes.
Farina V, Lepore G, Biagi F, Carcupino M, Zedda M. Farina V, et al. Eur J Histochem. 2018 Apr 3;62(2):2891. doi: 10.4081/ejh.2018.2891. Eur J Histochem. 2018. PMID: 29943951 Free PMC article. - Novel Drug Candidates Improve Ganglioside Accumulation and Neural Dysfunction in GM1 Gangliosidosis Models with Autophagy Activation.
Kajihara R, Numakawa T, Odaka H, Yaginuma Y, Fusaki N, Okumiya T, Furuya H, Inui S, Era T. Kajihara R, et al. Stem Cell Reports. 2020 May 12;14(5):909-923. doi: 10.1016/j.stemcr.2020.03.012. Epub 2020 Apr 16. Stem Cell Reports. 2020. PMID: 32302553 Free PMC article.
References
- Xie Z, Klionsky DJ. Autophagosome formation: core machinery and adaptations. Nat Cell Biol. 2007;9:1102–1109. - PubMed
- Novikoff AB. Electron microscopy: cytology of cell fractions. Science. 1956;124:969–972. - PubMed
- De Duve C, Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–492. - PubMed
- Dixon JS. “Phagocytic” lysosomes in chromatolytic neurones. Nature. 1967;215:657–658. - PubMed
- Rubinsztein DC, DiFiglia M, Heintz N, Nixon RA, Qin ZH, Ravikumar B, Stefanis L, Tolkovsky A. Autophagy and its possible roles in nervous system diseases, damage and repair. Autophagy. 2005;1:11–22. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical