Targeted electrode-based modulation of neural circuits for depression - PubMed (original) (raw)
Review
Targeted electrode-based modulation of neural circuits for depression
Helen S Mayberg. J Clin Invest. 2009 Apr.
Abstract
During the last 20 years of neuroscience research, we have witnessed a fundamental shift in the conceptualization of psychiatric disorders, with the dominant psychological and neurochemical theories of the past now complemented by a growing emphasis on developmental, genetic, molecular, and brain circuit models. Facilitating this evolving paradigm shift has been the growing contribution of functional neuroimaging, which provides a versatile platform to characterize brain circuit dysfunction underlying specific syndromes as well as changes associated with their successful treatment. Discussed here are converging imaging findings that established a rationale for testing a targeted neuromodulation strategy, deep brain stimulation, for treatment-resistant major depression.
Figures
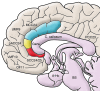
Figure 1. Anatomical locations of key brain regions implicated in MDD.
Midline, sagittal view of the brain, with subsections of the anterior cingulate cortex highlighted by coloring. A-Hc, amygdala hippocampus; BS, brainstem; C. callosum, corpus callosum; dMF9, dorso-medial frontal cortex BA9; MCC24, mid-cingulate cortex BA24 (blue); OF11, orbital frontal cortex BA11; pACC24, pregenual anterior cingulate cortex BA24 (yellow); PCC23, posterior cingulate cortex BA23; SCC24/25, SCC BA24 and BA25 (red); vMF10, ventro-medial frontal cortex BA10.
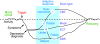
Figure 2. Theoretical time course of mood circuit changes during a depressive episode.
Functional neuroimaging abnormalities are viewed as the net effect of a triggering event and subsequent intrinsic adaptive or maladaptive responses, in other words, failure to self-correct. The nature of these compensatory changes is considered critical for understanding clinical symptom heterogeneity and clinical subtypes of MDD, providing a potential future framework for the development of brain-based algorithms for treatment selection based on distinct circuit patterns or brain phenotypes (indicated here as scan types i–iv). By example, scan type i, characterized by maladaptive overcorrection of the circuit, might be optimally treated with CBT. In contrast, failure to initiate or sustain any adaptive response, as defined here by scan type iv, might require ECT or DBS.
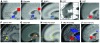
Figure 3. Converging evidence implicating the SCC region in MDD.
(A–E) Common pattern of changes in glucose metabolism or blood flow in the SCC with antidepressant response to various interventions. Images demonstrate group change patterns relative to the baseline depressed state for each treatment: (A) metabolic decreases with the selective serotonin reuptake inhibitor (SSRI) fluoxetine; (B) metabolic decreases with a placebo pill; (C) metabolic decreases with the serotonin-norepinephrine reuptake inhibitor (SNRI) venlafaxine; (D) blood flow decreases with ECT; and (E) metabolic increases with CBT. (F–J) Images demonstrate elevated resting-state SCC25 activity in various groups of patients with TRD: (F) metabolic increases in CBT and venlafaxine (V) nonresponders (NRs) relative to both healthy subjects and similarly depressed patients who responded to either treatment; (G) resting-state fMRI increases in pharmacotherapy (Med) nonresponders relative to healthy controls; (H) glucose metabolic increases in patients with TRD who later responded to cingulotomy (CGT) relative to those that failed to respond; (I) blood flow increases in patients with TRD, enrolled in a DBS treatment trial relative to healthy controls; (J) SCC blood flow increases with induction of transient sadness induced by recollection of a personal sad memory in healthy subjects, a pattern similar to that seen in patients with TRD. Red indicates increased activity (white arrows) and blue indicates decreased activity (black arrows). Images are courtesy of Mitch Nobler (D), Michael Greicius (G), and Darin Dougherty (H). Panels A and J are generated from data published in American Journal of Psychiatry (10). PanelsB and D are adapted with permission from_American Journal of Psychiatry_ (refs. and , respectively). Panel I is adapted with permission from_Neuron_ (11). PanelsC and E are adapted with permission from_American Journal of Psychiatry_ (62). Panel F is generated from data published in_American Journal of Psychiatry_ (62). Panel G is adapted with permission from_Biological Psychiatry_ (68). Panel H is adapted with permission from Journal of Neurosurgery (66).
Figure 4. Selective targeting of the depression circuit with DBS in patients with TRD.
(A) Preoperative (pre-op) MRI demonstrating the intended anatomical location for the DBS electrode within the SCC white matter. The four individual contacts on the electrode (numbered 1–4) can be independently stimulated using a programmable implanted pulse generator. (B) Preoperative blood flow PET scan demonstrating baseline hyperactivity of the SCC in the TRD study group (n = 6) relative to healthy controls. (C) The postoperative (post-op) MRI with the electrodes in place within the SCC white matter. (D) Six-month blood flow change relative to preoperative baseline associated with chronic DBS of the optimal SCC contact in four DBS responders. Red indicates increased blood flow and blue indicates decreased blood flow. hth, hypothalamus; MCC, mid-cingulate cortex; sn, substantia nigra; vCd, ventral caudate (adapted with permission from Neuron [ref. 11]).
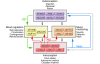
Figure 5. Circuit model of MDD.
Regions with known anatomical interconnections that show consistent changes across converging imaging experiments form the basis of this model. Regions are grouped into four main compartments, reflecting general behavioral dimensions of MDD and regional targets of various antidepressant treatments. Regions within a compartment all have strong anatomical connections to one another. Black arrows identify cross-compartment anatomical connections. Solid colored arrows identify putative connections between compartments mediating a specific treatment: green indicates CBT; blue indicates pharmacotherapy; red indicates SCC DBS. a-ins, anterior insula; amg, amygdala; dm-Th, dorsomedial thalamus; dp-Hc, dorsal-posterior hippocampus; mb-vta, midbrain-ventral tegmental area; mF10/9, medial frontal cortex BA10 and BA9; mOF11, medial orbital frontal cortex BA11; Par40, parietal cortex BA40; PF46/9, prefrontal cortex BA46 and BA9; PM6, premotor cortex BA6; va-HC, ventral-anterior hippocampus; vst-cd, ventral striatum-caudate.
Similar articles
- Multimodal approaches to define network oscillations in depression.
Smart OL, Tiruvadi VR, Mayberg HS. Smart OL, et al. Biol Psychiatry. 2015 Jun 15;77(12):1061-70. doi: 10.1016/j.biopsych.2015.01.002. Epub 2015 Jan 28. Biol Psychiatry. 2015. PMID: 25681871 Free PMC article. Review. - Uncovering the mechanisms of deep brain stimulation for Parkinson's disease through functional imaging, neural recording, and neural modeling.
McIntyre CC, Thakor NV. McIntyre CC, et al. Crit Rev Biomed Eng. 2002;30(4-6):249-81. doi: 10.1615/critrevbiomedeng.v30.i456.20. Crit Rev Biomed Eng. 2002. PMID: 12739751 Review. - Monoamine neurocircuitry in depression and strategies for new treatments.
Hamon M, Blier P. Hamon M, et al. Prog Neuropsychopharmacol Biol Psychiatry. 2013 Aug 1;45:54-63. doi: 10.1016/j.pnpbp.2013.04.009. Epub 2013 Apr 19. Prog Neuropsychopharmacol Biol Psychiatry. 2013. PMID: 23602950 Review. - Functional neurosurgery. The modulation of neural and mind circuits.
Al-Otaibi F, Al-Khairallah T. Al-Otaibi F, et al. Neurosciences (Riyadh). 2012 Jan;17(1):16-31. Neurosciences (Riyadh). 2012. PMID: 22246006 Review. - New methods of minimally invasive brain modulation as therapies in psychiatry: TMS, MST, VNS and DBS.
George MS. George MS. Zhonghua Yi Xue Za Zhi (Taipei). 2002 Aug;65(8):349-60. Zhonghua Yi Xue Za Zhi (Taipei). 2002. PMID: 12455803 Review.
Cited by
- Depression and metabolic connectivity: insights into the locus coeruleus, HF-rTMS, and anxiety.
Wu GR, Baeken C. Wu GR, et al. Transl Psychiatry. 2024 Nov 2;14(1):459. doi: 10.1038/s41398-024-03171-9. Transl Psychiatry. 2024. PMID: 39488540 Free PMC article. - [Critical alterations in the brain and psyche].
Brenner M, Durstewitz D. Brenner M, et al. Nervenarzt. 2024 Nov;95(11):1013-1023. doi: 10.1007/s00115-024-01770-x. Epub 2024 Oct 22. Nervenarzt. 2024. PMID: 39438289 Review. German. - Target engagement of the subgenual anterior cingulate cortex with transcranial temporal interference stimulation in major depressive disorder: a protocol for a randomized sham-controlled trial.
Demchenko I, Rampersad S, Datta A, Horn A, Churchill NW, Kennedy SH, Krishnan S, Rueda A, Schweizer TA, Griffiths JD, Boyden ES, Santarnecchi E, Bhat V. Demchenko I, et al. Front Neurosci. 2024 Aug 29;18:1390250. doi: 10.3389/fnins.2024.1390250. eCollection 2024. Front Neurosci. 2024. PMID: 39268031 Free PMC article. - Barriers and Facilitators for Participation in Brain Magnetic Resonance Imaging (MRI) Scans in Cancer Research: A Feasibility and Acceptability Analysis.
Manuweera T, Karunakaran K, Baechler C, Rosales J, Kleckner AS, Rosenblatt P, Ciner A, Kleckner IR. Manuweera T, et al. Res Sq [Preprint]. 2024 Jul 18:rs.3.rs-4595719. doi: 10.21203/rs.3.rs-4595719/v1. Res Sq. 2024. PMID: 39070661 Free PMC article. Preprint. - Blunted stimulus-preceding negativity during reward anticipation in major depressive disorder.
Ren X, White EJ, Nacke M, Mayeli A, Touthang J, Al Zoubi O, Kuplicki R, Victor TA, Paulus MP, Aupperle RL, Stewart JL. Ren X, et al. J Affect Disord. 2024 Oct 1;362:779-787. doi: 10.1016/j.jad.2024.07.060. Epub 2024 Jul 17. J Affect Disord. 2024. PMID: 39029684
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous