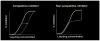A pièce de resistance: how HIV-1 escapes small molecule CCR5 inhibitors - PubMed (original) (raw)
Review
A pièce de resistance: how HIV-1 escapes small molecule CCR5 inhibitors
John P Moore et al. Curr Opin HIV AIDS. 2009 Mar.
Abstract
Purpose of review: Small molecule inhibitors targeting the CCR5 coreceptor represent a new class of drugs for treating HIV-1 infection. Maraviroc has received regulatory approvals, and vicriviroc is in phase 3 trials. Understanding how resistance to these drugs develops and is diagnosed is essential to guide clinical practice. We review what has been learned from in-vitro resistance studies, and how this relates to what is being seen, or can be anticipated, in clinical studies.
Recent findings: The principal resistance pathway in vitro involves continued use of CCR5 in an inhibitor-insensitive manner; the resistant viruses recognize the inhibitor-CCR5 complex, as well as free CCR5. Switching to use the CXCR4 coreceptor is rare. The principal genetic pathway involves accumulating 2-4 sequence changes in the gp120 V3 region, but a non-V3 pathway is also known. The limited information available from clinical studies suggests that a similar escape process is followed in vivo. However, the most common change associated with virologic failure involves expansion of pre-existing, CXCR4-using viruses that are insensitive to CCR5 inhibitors.
Summary: HIV-1 escapes small molecule CCR5 inhibitors by continuing to use CCR5 in an inhibitor-insensitive manner, or evades them by expanding naturally insensitive, CXCR4-using variants.
Figures
Figure 1. A commonly used assay for HIV-1 coreceptor usage
Plasmids expressing a HIV-1 Env glycoprotein and an Env-deleted HIV-1 genome with a linked luciferase reporter gene are cotransfected into a producer cell line (typically 293T cells). The resulting Env-pseudotyped viruses are collected and used to infect a target cell line that expresses CD4 and either CCR5 or CXCR4 (typically U87-CD4-CCR5 or U87-CD4-CXCR4 cells). Infection is quantified by the amount of luciferase activity present in the target cells. The commercially available Trofile and PhenoSense assays from Monogram Inc are based on this method [39]. This figure is adapted from [39].
Figure 2. Competitive and noncompetitive inhibition
With competitive inhibition (left), resistance is manifested by a rightwards shift in the dose response curve (dotted line) such that the IC50 value for the inhibitor increases. If enough inhibitor is present, the extent of inhibition can still reach 100%. With noncompetitive inhibition (right), resistance is manifested by a reduction in the extent of inhibition (dotted line) but without a change in IC50 value. Increasing the inhibitor concentration beyond the point at which inhibition is maximal (the maximum percentage inhibition, MPI, value) has no additional effect. Resistance to the small molecule CCR5 inhibitors is noncompetitive, sometimes referred to as allosteric [12••, 16••].
Similar articles
- V3 determinants of HIV-1 escape from the CCR5 inhibitors Maraviroc and Vicriviroc.
Berro R, Klasse PJ, Jakobsen MR, Gorry PR, Moore JP, Sanders RW. Berro R, et al. Virology. 2012 Jun 5;427(2):158-65. doi: 10.1016/j.virol.2012.02.006. Epub 2012 Mar 16. Virology. 2012. PMID: 22424737 Free PMC article. - Characterizing the Diverse Mutational Pathways Associated with R5-Tropic Maraviroc Resistance: HIV-1 That Uses the Drug-Bound CCR5 Coreceptor.
Jiang X, Feyertag F, Meehan CJ, McCormack GP, Travers SA, Craig C, Westby M, Lewis M, Robertson DL. Jiang X, et al. J Virol. 2015 Nov;89(22):11457-72. doi: 10.1128/JVI.01384-15. Epub 2015 Sep 2. J Virol. 2015. PMID: 26339063 Free PMC article. - A maraviroc-resistant HIV-1 with narrow cross-resistance to other CCR5 antagonists depends on both N-terminal and extracellular loop domains of drug-bound CCR5.
Tilton JC, Wilen CB, Didigu CA, Sinha R, Harrison JE, Agrawal-Gamse C, Henning EA, Bushman FD, Martin JN, Deeks SG, Doms RW. Tilton JC, et al. J Virol. 2010 Oct;84(20):10863-76. doi: 10.1128/JVI.01109-10. Epub 2010 Aug 11. J Virol. 2010. PMID: 20702642 Free PMC article. - [Mechanisms of resistance and failure of treatment with maraviroc].
Delgado R. Delgado R. Enferm Infecc Microbiol Clin. 2008 Oct;26 Suppl 11:28-33. doi: 10.1016/s0213-005x(08)76561-3. Enferm Infecc Microbiol Clin. 2008. PMID: 19133219 Review. Spanish. - [Conclusions and perspectives. Maraviroc].
Alcamí J. Alcamí J. Enferm Infecc Microbiol Clin. 2008 Oct;26 Suppl 11:49-54. doi: 10.1016/s0213-005x(08)76564-9. Enferm Infecc Microbiol Clin. 2008. PMID: 19133222 Review. Spanish.
Cited by
- Dopamine Levels Induced by Substance Abuse Alter Efficacy of Maraviroc and Expression of CCR5 Conformations on Myeloid Cells: Implications for NeuroHIV.
Matt SM, Nickoloff-Bybel EA, Rong Y, Runner K, Johnson H, O'Connor MH, Haddad EK, Gaskill PJ. Matt SM, et al. Front Immunol. 2021 May 19;12:663061. doi: 10.3389/fimmu.2021.663061. eCollection 2021. Front Immunol. 2021. PMID: 34093554 Free PMC article. - Multiple ways of targeting APOBEC3-virion infectivity factor interactions for anti-HIV-1 drug development.
Smith JL, Bu W, Burdick RC, Pathak VK. Smith JL, et al. Trends Pharmacol Sci. 2009 Dec;30(12):638-46. doi: 10.1016/j.tips.2009.09.006. Trends Pharmacol Sci. 2009. PMID: 19837465 Free PMC article. Review. - Natural anti-CCR5 antibodies in HIV-infection and -exposure.
Lopalco L. Lopalco L. J Transl Med. 2011 Jan 27;9 Suppl 1(Suppl 1):S4. doi: 10.1186/1479-5876-9-S1-S4. J Transl Med. 2011. PMID: 21284903 Free PMC article. Review. - Cell-type specific requirements for thiol/disulfide exchange during HIV-1 entry and infection.
Stantchev TS, Paciga M, Lankford CR, Schwartzkopff F, Broder CC, Clouse KA. Stantchev TS, et al. Retrovirology. 2012 Dec 3;9:97. doi: 10.1186/1742-4690-9-97. Retrovirology. 2012. PMID: 23206338 Free PMC article. - A model of peptide triazole entry inhibitor binding to HIV-1 gp120 and the mechanism of bridging sheet disruption.
Emileh A, Tuzer F, Yeh H, Umashankara M, Moreira DR, Lalonde JM, Bewley CA, Abrams CF, Chaiken IM. Emileh A, et al. Biochemistry. 2013 Apr 2;52(13):2245-61. doi: 10.1021/bi400166b. Epub 2013 Mar 22. Biochemistry. 2013. PMID: 23470147 Free PMC article.
References
- Kuhmann SE, Hartley O. Targeting chemokine receptors in HIV: a status report. Annu Rev Pharmacol Toxicol. 2008;48:425–461. A recent review of the preclinical and clinical development of CCR5 and CXCR4 inhibitors as therapies for HIV-1 infection. - PubMed
- Tsibris AMN, Kuritzkes DR. Chemokine antagonists as therapeutics: focus on HIV-1. Annu Rev Med. 2007;58:13–15. A recent review of the preclinical and clinical development of CCR5 and CXCR4 inhibitors as therapies for HIV-1 infection. - PubMed
- MacArthur RD, Novak RM. Reviews of antiinfective agents: maraviroc: the first of a new class of antiretroviral agents. Clin Infect Dis. 2008;47:236–241. - PubMed
- Billick E, Seibert C, Pugach P, et al. The differential sensitivity of human and rhesus macaque CCR5 to small-molecule inhibitors of human immunodeficiency virus type 1 entry is explained by a single amino acid difference and suggests a mechanism of action for these inhibitors. J Virol. 2004;78:4134–4144. - PMC - PubMed
- Castonguay LA, Weng Y, Adolfsen W, et al. Binding of 2-aryl-4-(piperidin-1-yl)butanamines and 1,3,4-trisubstituted pyrrolidines to human CCR5: a molecular modeling-guided mutagenesis study of the binding pocket. Biochemistry. 2003;42:1544–1550. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI041420/AI/NIAID NIH HHS/United States
- R37 AI 55357/AI/NIAID NIH HHS/United States
- R01 AI041420-12/AI/NIAID NIH HHS/United States
- R01 AI 41420/AI/NIAID NIH HHS/United States
- R37 AI055357/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials