Langerhans cells are not required for the CD8 T cell response to epidermal self-antigens - PubMed (original) (raw)
Langerhans cells are not required for the CD8 T cell response to epidermal self-antigens
Laura S Bursch et al. J Immunol. 2009.
Abstract
Langerhans cells (LC) are APC that reside at the barrier surfaces. Mice expressing an OVA peptide in the epidermis (K14-OVAp) were used to study CD8(+) T cell responses to an epidermal self-Ag. Earlier results suggested that LC were the predominant APC, inducing a robust T cell response and autoimmunity. In this study, we used a whole protein model system, the K14-mOVA mouse, in which a transmembrane form of OVA was expressed in keratinocytes. In contrast to K14-OVAp mice, T cells in K14-mOVA mice were activated, but did not expand and instead died by apoptosis. Furthermore, in double-transgenic mice expressing both mOVA and OVAp, robust OT-I expansion occurred, indicating that tolerance to this Ag is not dominant and was due to lack of activating signals. We sought to identify the relevant APC in K14 mice using bone marrow chimeras and found that radioresistant cells (presumably LC) were able to cross-present the OVA Ag from keratinocytes to naive T cells in the lymph node. However, use of LC-deficient mice indicated that LC were not required for the expansion of OT-I in K14-OVAp or the deletion of OT-I in K14-mOVA mice. These data suggest that radioresistant non-LC present self-Ag in K14-OVAp mice and drive a robust CD8 T cell response.
Conflict of interest statement
Disclosures: The authors declare no conflict of interest or financial interests
Figures
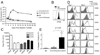
Figure 1. Distinct outcomes of OT-I T cells adoptively transferred into K14-OVAp and K14-mOVA mice
Naïve OT-I T cells were adoptively transferred into K14-OVAp, K14-mOVA, or B6 mice. Lymph nodes and spleen were harvested and analyzed by flow cytometry. (A) Fold expansion of 5 × 104 OT-I in different hosts at varying timepoints after adoptive transfer. The total number of OT-I was calculated from LN and spleen. Data shown is the average of at least 2 mice per group per timepoint. Inset depicts CFSE dilution in OT-I in K14-mOVA and B6 mice at day 4. (B) Increasing the number of adoptively transferred OT-I in K14-mOVA did not increase expansion observed at day 6. Data are from least 4 mice per group compiled from two experiments. (C) Phenotyptic analysis of LN cells 1 or 2 days after adoptive transfer of 1 × 106 naïve OT-I. Adoptively transferred OT-I (CD8a+, Thy1.1+) were gated and analyzed for CD25 (IL-2Rα), 4-1BB, CD54, and CD27. Data shown are representative of cells in both LN and spleen, in at least 4 mice per group from two experiments. (D) Intracellular staining for cleaved caspase 3 on OT-I T cells recovered from the lymph nodes at day 2 following adoptive transfer. Data shown are from a total of 4 mice per group in two experiments.
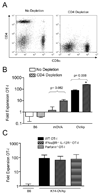
Figure 2. The robust OT-I response in K14-OVAp does not require CD4 help or cytolytic effector function
(A) Naïve OT-I T cells were adoptively transferred into K14-OVAp, K14-mOVA, or B6 mice that were treated with GK1.5 (to deplete CD4 T cells) or control antibody. Lymph nodes and spleen were harvested at day 6 and analyzed by flow cytometry. Staining of LN cells from GK1.5 treated mice with a non-competing CD4 antibody showed effective depletion of CD4 T cells from the lymphoid organs of recipient mice. Representative data are shown of 10 depleted mice. (B) Expansion of adoptively transferred OT-I in CD4 depleted B6, K14-mOVA, or K14-OVAp mice. Data shown include a total of at least 3 mice per group from two experiments. (C) Fold expansion of IFNα/βR−/− IL-12R−/− OT-I or Perforin−/− OT-I after adoptive transfer into K14-OVAp recipient mice. Data shown are representative of a total of 6 mice per group from two experiments.
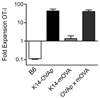
Figure 3. OT-I expansion is dominant in K14-OVAp×K14-mOVA double transgenic mice
Naïve OT-I T cells were adoptively transferred into B6, K14-OVAp, K14-mOVA, or K14-OVAp/K14-mOVA double transgenic mice. Lymph nodes and spleen were harvested at day 6 and analyzed by flow cytometry. Data shown are from a total of 5 mice per group from two experiments.
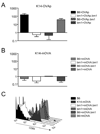
Figure 4. Both radioresistant and hematopoetic cells present antigen in K14-OVAp and K14-mOVA mice, but only radioresistant cells drive expansion in K14-OVAp
Bone marrow chimeras were made using the indicated donors and hosts. Naïve OT-I T cells were adoptively transferred into K14-OVAp (A) and K14-mOVA (B) chimeras. Fold expansion of OT-I at day 6 is shown, and includes 2–6 mice per group from 2 experiments. (C) OT-I T cells adoptively were transferred into K14-mOVA chimeras and analyzed 2 days later. Data is representative of 3 chimeras per group from two experiments.
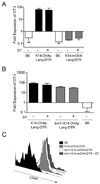
Figure 5. Langerhans cells can present K14 antigens, but are not required for activation in either K14-OVAp or K14-mOVA mice
(A) Naïve OT-I T cells were adoptively transferred into K14-OVAp/Lang-DTR and K14-mOVA/Lang-DTR mice that had been treated with 1µg diphtheria toxin i.p. at day −4, −1 and +2 relative to adoptive transfer. Lymph nodes and spleen were harvested at day 6 and analyzed by flow cytometry. Depletion of LC did not affect expansion in K14-OVAp or K14-mOVA. Data shown include 3–6 mice per group from two experiments. (B–C) Bone marrow chimeras were made using the H-2Kbm1 bone marrow donors and K14-OVAp/Lang-DTR and K14-mOVA/Lang-DTR recipients. 1µg diphtheria toxin (DT) was administered i.p. on day −4, −1 and +2 relative to adoptive transfer of OT-I. (B) Naïve OT-I T cells were adoptively transferred into DT treated or control K14-OVAp/Lang-DTR mice and chimeras. Lymph nodes and spleen were harvested at day 6 and analyzed by flow cytometry. Data shown includes 3–6 mice per group in two experiments. (C) Naïve OT-I T cells were adoptively transferred into DT treated or control K14-mOVA/Lang-DTR mice and chimeras. Lymph nodes and spleen were harvested at day 3 and analyzed by flow cytometry. Histograms are representative of at least 4 mice per group from two experiments.
Similar articles
- The use of mouse models to better understand mechanisms of autoimmunity and tolerance.
Miyagawa F, Gutermuth J, Zhang H, Katz SI. Miyagawa F, et al. J Autoimmun. 2010 Nov;35(3):192-8. doi: 10.1016/j.jaut.2010.06.007. Epub 2010 Jul 23. J Autoimmun. 2010. PMID: 20655706 Free PMC article. Review. - Induction of GVHD-like skin disease by passively transferred CD8(+) T-cell receptor transgenic T cells into keratin 14-ovalbumin transgenic mice.
Shibaki A, Sato A, Vogel JC, Miyagawa F, Katz SI. Shibaki A, et al. J Invest Dermatol. 2004 Jul;123(1):109-15. doi: 10.1111/j.0022-202X.2004.22701.x. J Invest Dermatol. 2004. PMID: 15191550 - Keratinocytes function as accessory cells for presentation of endogenous antigen expressed in the epidermis.
Kim BS, Miyagawa F, Cho YH, Bennett CL, Clausen BE, Katz SI. Kim BS, et al. J Invest Dermatol. 2009 Dec;129(12):2805-17. doi: 10.1038/jid.2009.176. Epub 2009 Jun 25. J Invest Dermatol. 2009. PMID: 19554018 Free PMC article. - IL-15 serves as a costimulator in determining the activity of autoreactive CD8 T cells in an experimental mouse model of graft-versus-host-like disease.
Miyagawa F, Tagaya Y, Kim BS, Patel HJ, Ishida K, Ohteki T, Waldmann TA, Katz SI. Miyagawa F, et al. J Immunol. 2008 Jul 15;181(2):1109-19. doi: 10.4049/jimmunol.181.2.1109. J Immunol. 2008. PMID: 18606663 Free PMC article. - Cross-presentation of self antigens to CD8+ T cells: the balance between tolerance and autoimmunity.
Kurts C, Heath WR, Carbone FR, Kosaka H, Miller JF. Kurts C, et al. Novartis Found Symp. 1998;215:172-81; discussion 181-90. doi: 10.1002/9780470515525.ch13. Novartis Found Symp. 1998. PMID: 9760579 Review.
Cited by
- TLR7 enables cross-presentation by multiple dendritic cell subsets through a type I IFN-dependent pathway.
Oh JZ, Kurche JS, Burchill MA, Kedl RM. Oh JZ, et al. Blood. 2011 Sep 15;118(11):3028-38. doi: 10.1182/blood-2011-04-348839. Epub 2011 Aug 2. Blood. 2011. PMID: 21813451 Free PMC article. - The use of mouse models to better understand mechanisms of autoimmunity and tolerance.
Miyagawa F, Gutermuth J, Zhang H, Katz SI. Miyagawa F, et al. J Autoimmun. 2010 Nov;35(3):192-8. doi: 10.1016/j.jaut.2010.06.007. Epub 2010 Jul 23. J Autoimmun. 2010. PMID: 20655706 Free PMC article. Review. - Murine Langerin+ dermal dendritic cells prime CD8+ T cells while Langerhans cells induce cross-tolerance.
Flacher V, Tripp CH, Mairhofer DG, Steinman RM, Stoitzner P, Idoyaga J, Romani N. Flacher V, et al. EMBO Mol Med. 2014 Sep;6(9):1191-204. doi: 10.15252/emmm.201303283. EMBO Mol Med. 2014. PMID: 25085878 Free PMC article. - Cross-presentation by dendritic cells.
Joffre OP, Segura E, Savina A, Amigorena S. Joffre OP, et al. Nat Rev Immunol. 2012 Jul 13;12(8):557-69. doi: 10.1038/nri3254. Nat Rev Immunol. 2012. PMID: 22790179 Review. - Dendritic cell migration limits the duration of CD8+ T-cell priming to peripheral viral antigen.
Schell AM, Granger EL, Koczot F, Fischer MA, Norbury CC. Schell AM, et al. J Virol. 2010 Apr;84(7):3586-94. doi: 10.1128/JVI.01975-09. Epub 2010 Jan 20. J Virol. 2010. PMID: 20089641 Free PMC article.
References
- Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. - PubMed
- Kishimoto H, Sprent J. A defect in central tolerance in NOD mice. Nat Immunol. 2001;2:1025–1031. - PubMed
- Bouneaud C, Kourilsky P, Bousso P. Impact of negative selection on the T cell repertoire reactive to a self-peptide: a large fraction of T cell clones escapes clonal deletion. Immunity. 2000;13:829–840. - PubMed
- Ohashi PS, Oehen S, Aichele P, Pircher H, Odermatt B, Herrera P, Higuchi Y, Buerki K, Hengartner H, Zinkernagel RM. Induction of diabetes is influenced by the infectious virus and local expression of MHC class I and tumor necrosis factor-alpha. J Immunol. 1993;150:5185–5194. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI35296/AI/NIAID NIH HHS/United States
- P01 AI035296/AI/NIAID NIH HHS/United States
- AI70380/AI/NIAID NIH HHS/United States
- U01 AI070380-03/AI/NIAID NIH HHS/United States
- U01 AI070380/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials