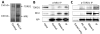Mili interacts with tudor domain-containing protein 1 in regulating spermatogenesis - PubMed (original) (raw)
Mili interacts with tudor domain-containing protein 1 in regulating spermatogenesis
Jianquan Wang et al. Curr Biol. 2009.
Abstract
Piwi proteins are essential for germline development, stem cell self-renewal, epigenetic regulation, and transposon silencing [1-7]. They bind to a complex class of small noncoding RNAs called Piwi-interacting RNAs (piRNAs) [8]. Mammalian Piwi proteins such as Mili are localized in the cytoplasm of spermatogenic cells, where they are associated with a germline-specific organelle called the nuage or its derivative, the chromatoid body, as well as with polysomes [9]. To investigate the molecular mechanisms mediated by Mili, we searched for Mili-interacting proteins. Here, we report that Mili specifically interacts with Tudor domain-containing protein 1 (Tdrd1), a germline protein that contains multiple Tudor domains [10, 11]. This RNA-independent interaction is mediated through the N-terminal domain of Mili and the N-terminal region of Tdrd1 containing the myeloid Nervy DEAF-1 (MYND) domain and the first two Tudor domains. In addition, Mili positively regulates the expression of the Tdrd1 mRNA. Furthermore, Mili and Tdrd1 mutants share similar spermatogenic defects. However, Tdrd1, unlike Mili, is not required for piRNA biogenesis. Our results suggest that Mili interacts with Tdrd1 in the nuage and chromatoid body. This interaction does not contribute to piRNA biogenesis but represents a regulatory mechanism that is critical for spermatogenesis.
Figures
Figure 1. Mili specifically interacts with Tdrd1 in an RNA-independent manner
A, Silver-stained SDS-PAGE gels revealing the products of Mili antibody immunoprecipitation of testicular extract. The immunoprecipitation in the presence of blocking peptide was used as negative controls. Two specific bands (~100 and 140kDa) were identified as Mili and Tdrd1 by mass spectrometry. B, Western blot showing that Tdrd1 was co-immunoprecipitated by Mili antibody. The Mili-Tdrd1 interaction is RNA-independent. Peptide-blocked Mili antibody was used as a negative control. IgG band was used as loading control. C, Western blot showing that Mili was co-immunoprecipitated by Tdrd1 antibody. Extract plus beads, and antibody plus beads were used as negative control for IP. The Mili-Tdrd1 interaction is RNA-independent. IgG band was used as loading control.
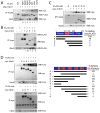
Figure 2. The specificity and domains of Mili-Tdrd1 interaction
A, Mili preferentially interacts with Tdrd1. 293T cells were transfected with indicated FLAG plasmids and myc-tagged Tdrd1, followed by immunoprecipitation with FLAG beads and subjected to western blotting. The numbers under the blots represent the myc/FLAG signal in the IP FLAG blots. B, Tdrd1 preferentially interacts with Mili. 293T cells were transfected with FLAG-tagged Mili and constructs encoding the indicated myc-taged Tdrd protein, followed by immunoprecipitation with myc antibodies and subjected to western blotting. C, The N-terminal portion of Mili mediates its interaction with Tdrd1. Indicated FLAG-tagged Mili deletion constructs and myc-tagged Tdrd1 were transfected into 293T cells and analyzed as described in (A). D, Multiple domains of Tdrd1 are required for interaction with Mili. Indicated myc-tagged Tdrd1 deletion constructs were transfected into 293T cells with FLAG-tagged Mili and analyzed as described in (B). * indicates either N-terminal or C-terminal myc-tag, m=zinc finger MynD domain.
Figure 3. Mili colocalizes with Tdrd1 in cytoplasm and in the nuage and chromatoid body
A, Western blots showing that Tdrd1 and Mili are primarily expressed in cytoplasm. Purity of the fractionations was confirmed by western blotting for GAPDH as a cytoplasmic marker and RNA PolII as a nuclear marker. B, Tdrd1 (green) was localized in the cytoplasm and enriched in the dense granule/nuage in spermatogonia and spermatocytes and chromatoid body of spermatids. Mili (red) co-localized with Tdrd1 in the cytoplasm, especially in these granules. DNA was stained with DAPI (as shown in blue). C, Western blotting of Tdrd1 in 13 dpp mili mutant showing that cytoplasmic Tdrd1 is drastically reduced in the mili mutant. D, In 13 dpp mili+/+ testes, Tdrd1 is primarily localized to the cytoplasm of spermatogonia and spermatocytes. While in 13 dpp _mili_-/- testes show drastic loss of cytoplasmic Tdrd1 in spermatogonia and spermatocytes. The bars are 25μm.
Figure 4. The interaction between Mili and Tdrd1 is not related to translation and piRNA biogenesis
A, Tdrd1 and Mili fraction profile of two-month old wildtype testis in a 15-50% (W/W) sucrose gradient. B and C, fractionation profiles of wildtype testis following treatment with EDTA and micrococcal nuclease respectively. D, Mili distribution on polysome fractions in _tdrd_-/- testes. E, a polyacrylamide gel showing piRNAs are present in similar abundance in Tdrd1+/+. Tdrd1+/-, and _Tdrd1_-/- testes. F, A working model on the interaction of Mili, Tdrd1, and other proteins in the nuage and chromatoid body, which is distinct from the Mili-eIF3a interactions in the translational machinery in the cytosol.
Similar articles
- Loss of the Mili-interacting Tudor domain-containing protein-1 activates transposons and alters the Mili-associated small RNA profile.
Reuter M, Chuma S, Tanaka T, Franz T, Stark A, Pillai RS. Reuter M, et al. Nat Struct Mol Biol. 2009 Jun;16(6):639-46. doi: 10.1038/nsmb.1615. Epub 2009 May 24. Nat Struct Mol Biol. 2009. PMID: 19465913 - Associations between PIWI proteins and TDRD1/MTR-1 are critical for integrated subcellular localization in murine male germ cells.
Kojima K, Kuramochi-Miyagawa S, Chuma S, Tanaka T, Nakatsuji N, Kimura T, Nakano T. Kojima K, et al. Genes Cells. 2009 Oct;14(10):1155-65. doi: 10.1111/j.1365-2443.2009.01342.x. Epub 2009 Sep 7. Genes Cells. 2009. PMID: 19735482 - The multiple Tudor domain-containing protein TDRD1 is a molecular scaffold for mouse Piwi proteins and piRNA biogenesis factors.
Mathioudakis N, Palencia A, Kadlec J, Round A, Tripsianes K, Sattler M, Pillai RS, Cusack S. Mathioudakis N, et al. RNA. 2012 Nov;18(11):2056-72. doi: 10.1261/rna.034181.112. Epub 2012 Sep 20. RNA. 2012. PMID: 22996915 Free PMC article. - piRNA and spermatogenesis in mice.
Chuma S, Nakano T. Chuma S, et al. Philos Trans R Soc Lond B Biol Sci. 2013 Jan 5;368(1609):20110338. doi: 10.1098/rstb.2011.0338. Philos Trans R Soc Lond B Biol Sci. 2013. PMID: 23166399 Free PMC article. Review. - Mitochondria Associated Germinal Structures in Spermatogenesis: piRNA Pathway Regulation and Beyond.
Wang X, Lv C, Guo Y, Yuan S. Wang X, et al. Cells. 2020 Feb 10;9(2):399. doi: 10.3390/cells9020399. Cells. 2020. PMID: 32050598 Free PMC article. Review.
Cited by
- Transposon Reactivation in the Germline May Be Useful for Both Transposons and Their Host Genomes.
Maupetit-Mehouas S, Vaury C. Maupetit-Mehouas S, et al. Cells. 2020 May 8;9(5):1172. doi: 10.3390/cells9051172. Cells. 2020. PMID: 32397241 Free PMC article. Review. - Mammalian piRNAs: Biogenesis, function, and mysteries.
Fu Q, Wang PJ. Fu Q, et al. Spermatogenesis. 2014 Feb 7;4:e27889. doi: 10.4161/spmg.27889. eCollection 2014. Spermatogenesis. 2014. PMID: 25077039 Free PMC article. Review. - Nonsense in the testis: multiple roles for nonsense-mediated decay revealed in male reproduction.
MacDonald CC, Grozdanov PN. MacDonald CC, et al. Biol Reprod. 2017 May 1;96(5):939-947. doi: 10.1093/biolre/iox033. Biol Reprod. 2017. PMID: 28444146 Free PMC article. Review. - Domestic chickens activate a piRNA defense against avian leukosis virus.
Sun YH, Xie LH, Zhuo X, Chen Q, Ghoneim D, Zhang B, Jagne J, Yang C, Li XZ. Sun YH, et al. Elife. 2017 Apr 6;6:e24695. doi: 10.7554/eLife.24695. Elife. 2017. PMID: 28384097 Free PMC article. - Heterotypic piRNA Ping-Pong requires qin, a protein with both E3 ligase and Tudor domains.
Zhang Z, Xu J, Koppetsch BS, Wang J, Tipping C, Ma S, Weng Z, Theurkauf WE, Zamore PD. Zhang Z, et al. Mol Cell. 2011 Nov 18;44(4):572-84. doi: 10.1016/j.molcel.2011.10.011. Mol Cell. 2011. PMID: 22099305 Free PMC article.
References
- Cox DN, Chao A, Lin H. piwi encodes a nucleoplasmic factor whose activity modulates the number and division rate of germline stem cells. Development (Cambridge, England) 2000;127:503–514. - PubMed
- Lin H, Spradling AC. A novel group of pumilio mutations affects the asymmetric division of germline stem cells in the Drosophila ovary. Development (Cambridge, England) 1997;124:2463–2476. - PubMed
- Pal-Bhadra M, Leibovitch BA, Gandhi SG, Rao M, Bhadra U, Birchler JA, Elgin SC. Heterochromatic silencing and HP1 localization in Drosophila are dependent on the RNAi machinery. Science (New York, N Y) 2004;303:669–672. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials