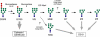Human OS-9, a lectin required for glycoprotein endoplasmic reticulum-associated degradation, recognizes mannose-trimmed N-glycans - PubMed (original) (raw)
Human OS-9, a lectin required for glycoprotein endoplasmic reticulum-associated degradation, recognizes mannose-trimmed N-glycans
Nobuko Hosokawa et al. J Biol Chem. 2009.
Abstract
In the endoplasmic reticulum (ER), lectins and processing enzymes are involved in quality control of newly synthesized proteins for productive folding as well as in the ER-associated degradation (ERAD) of misfolded proteins. ER quality control requires the recognition and modification of the N-linked oligosaccharides attached to glycoproteins. Mannose trimming from the N-glycans plays an important role in targeting of misfolded glycoproteins for ERAD. Recently, two mammalian lectins, OS-9 and XTP3-B, which contain mannose 6-phosphate receptor homology domains, were reported to be involved in ER quality control. Here, we examined the requirement for human OS-9 (hOS-9) lectin activity in degradation of the glycosylated ERAD substrate NHK, a genetic variant of alpha1-antitrypsin. Using frontal affinity chromatography, we demonstrated that the recombinant hOS-9 mannose 6-phosphate receptor homology domain specifically binds N-glycans lacking the terminal mannose from the C branch in vitro. To examine the specificity of OS-9 recognition of N-glycans in vivo, we modified the oligosaccharide structures on NHK by overexpressing ER alpha1,2-mannosidase I or EDEM3 and examined the effect of these modifications on NHK degradation in combination with small interfering RNA-mediated knockdown of hOS-9. The ability of hOS-9 to enhance glycoprotein ERAD depended on the N-glycan structures on NHK, consistent with the frontal affinity chromatography results. Thus, we propose a model for mannose trimming and the requirement for hOS-9 lectin activity in glycoprotein ERAD in which N-glycans lacking the terminal mannose from the C branch are recognized by hOS-9 and targeted for degradation.
Figures
FIGURE 1.
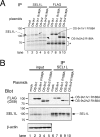
The hOS-9 lectin domain is required for ERAD of the misfolded glycoprotein NHK. A, inhibition of NHK degradation by hOS-9 knockdown. HEK 293 cells incubated with the indicated siRNA (30 n
m
) were pulse-labeled with [35S]methionine/cysteine for 15 min and chased for the indicated times. NHK was immunoprecipitated with anti-α1AT, and samples were separated by SDS-PAGE. The relative radioactivity of NHK (arrowhead) was quantified and normalized using the level at the 0 h chase as 100%. Error bars indicate S.E. (n = 3 or 4). Two negative control siRNAs (Control-1 and -2) and two specific siRNAs (OS9-2 and OS9-3) were used. B, degradation of NHK in the presence of the overexpressed hOS-9v1 R188A mutant. Cells were pulse-labeled as in A. Cell lysates were divided into aliquots and immunoprecipitated (IP) with anti-α1AT (lanes 1–9) or anti-FLAG (lanes 10–18). The arrowhead indicates the position of NHK, and the arrow indicates the position of the hOS-9v1-FLAG wild-type or R188A mutant. The asterisk denotes a hOS-9 fragment generated by overexpressing FLAG-tagged hOS-9. NHK was quantified and the data are presented in the panel on the right as the mean ± S.E. (n = 3). C, degradation of NHK-QQQ (black arrow) in the presence of the overexpressed hOS-9v1 or its R188A mutant. Quantitation is shown in the panel on the right.
FIGURE 2.
Co-immunoprecipitation of hOS-9 with SEL1L. A, co-immunoprecipitation of hOS-9 wild-type or the R188A mutant with SEL1L. HEK 293 cells were transfected with hOS-9v1-FLAG, v2-FLAG, or their respective R188A mutants, labeled with 35S-Protein Labeling mixture for 3 h, and immunoprecipitated with anti-SEL1L (lanes 1–5) or anti-FLAG (lanes 6–10). The asterisk indicates a protein non-specifically precipitated by the protein A- or protein G-Sepharose beads. B, co-immunoprecipitation of hOS-9 or the hOS-9 R188A mutant with SEL1L was detected by immunoprecipitation (IP) followed by Western blot analysis. Cells were harvested in a buffer containing 3% digitonin, immunoprecipitated with anti-SEL1L, blotted, and then probed with anti-FLAG or anti-SEL1L (lanes 6–10). Aliquots (1/10 volume) of the cell lysate used for immunoprecipitation were Western-blotted to detect transfected hOS-9 and endogenous SEL1L (lanes 1–5). The lower panel shows β-actin as a loading control.
FIGURE 3.
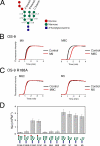
The hOS-9 MRH domain interacts with _N_-glycans lacking the terminal mannose from the C branch. A, the structure of the _N_-linked glycan G3M9 is shown schematically. The glycosidic linkages between mannose residues in each branch are indicated. B, elution profiles of the PA-oligosaccharides M9 and M8C from an affinity column presenting the hOS-9 MRH domain (red lines). The elution profile of a negative control glycan (PA-GD1b-hexasaccharide) is shown by the black line. C, elution profiles of the PA-oligosaccharides M8C and M5 from affinity columns presenting the MRH domain of the R188A mutant. Elution profiles of M8C and M5 are indicated by red lines, and that of a negative control glycan is shown by the black line. D, the affinity of the recombinant hOS-9 MRH domain for each oligosaccharide, as indicated by the Ka value, is presented as the mean ± S.D. (n = 3). G1M9, Glc1Man9GlcNAc2; M8A, Man8GlcNAc2 isomer A.
FIGURE 4.
Oligosaccharide structures modified by the overexpression of ER ManI or EDEM3 affect the degradation of NHK in hOS-9 knockdown cells. A, ER ManI overexpression. Shown are oligosaccharides on NHK after a 1-h chase after pulse-labeling with [3H]mannose in HEK 293 cells overexpressing ER ManI (left panel). NHK degradation in ER ManI-overexpressing cells treated with control or hOS-9 siRNA (middle panel) are shown. The data were quantified and presented as mean ± S.E. (n = 3 or 4) (right panel). B, EDEM3 overexpression. Oligosaccharide structures on NHK and NHK degradation were analyzed and quantified as in A. IP, immunoprecipitation; K/D, knockdown.
FIGURE 5.
Model for hOS-9 recognition of oligosaccharides on ERAD substrates in vivo. _N_-Glycan processing by different enzymes and recognition of the ERAD substrate by the ER lectins calnexin/calreticulin and hOS-9 under physiological conditions in vivo are illustrated. _N_-Glycans that lack the terminal mannose from the C branch, most likely M7, M6, and M5, are recognized by hOS-9 and targeted for degradation. G1M9, Glc1Man9GlcNAc2.
Similar articles
- Endoplasmic reticulum lectin XTP3-B inhibits endoplasmic reticulum-associated degradation of a misfolded α1-antitrypsin variant.
Fujimori T, Kamiya Y, Nagata K, Kato K, Hosokawa N. Fujimori T, et al. FEBS J. 2013 Mar;280(6):1563-75. doi: 10.1111/febs.12157. Epub 2013 Feb 28. FEBS J. 2013. PMID: 23356641 - Mannose trimming is required for delivery of a glycoprotein from EDEM1 to XTP3-B and to late endoplasmic reticulum-associated degradation steps.
Groisman B, Shenkman M, Ron E, Lederkremer GZ. Groisman B, et al. J Biol Chem. 2011 Jan 14;286(2):1292-300. doi: 10.1074/jbc.M110.154849. Epub 2010 Nov 9. J Biol Chem. 2011. PMID: 21062743 Free PMC article. - EDEM1 accelerates the trimming of alpha1,2-linked mannose on the C branch of N-glycans.
Hosokawa N, Tremblay LO, Sleno B, Kamiya Y, Wada I, Nagata K, Kato K, Herscovics A. Hosokawa N, et al. Glycobiology. 2010 May;20(5):567-75. doi: 10.1093/glycob/cwq001. Epub 2010 Jan 11. Glycobiology. 2010. PMID: 20065073 - Mannose 6-phosphate receptor homology domain-containing lectins in mammalian endoplasmic reticulum-associated degradation.
Hosokawa N, Kato K, Kamiya Y. Hosokawa N, et al. Methods Enzymol. 2010;480:181-97. doi: 10.1016/S0076-6879(10)80010-2. Methods Enzymol. 2010. PMID: 20816211 Review. - Quality control of glycoprotein folding and ERAD: the role of N-glycan handling, EDEM1 and OS-9.
Roth J, Zuber C. Roth J, et al. Histochem Cell Biol. 2017 Feb;147(2):269-284. doi: 10.1007/s00418-016-1513-9. Epub 2016 Nov 1. Histochem Cell Biol. 2017. PMID: 27803995 Review.
Cited by
- Deglycosylation-dependent fluorescent proteins provide unique tools for the study of ER-associated degradation.
Grotzke JE, Lu Q, Cresswell P. Grotzke JE, et al. Proc Natl Acad Sci U S A. 2013 Feb 26;110(9):3393-8. doi: 10.1073/pnas.1300328110. Epub 2013 Feb 11. Proc Natl Acad Sci U S A. 2013. PMID: 23401531 Free PMC article. - Road to ruin: targeting proteins for degradation in the endoplasmic reticulum.
Smith MH, Ploegh HL, Weissman JS. Smith MH, et al. Science. 2011 Nov 25;334(6059):1086-90. doi: 10.1126/science.1209235. Science. 2011. PMID: 22116878 Free PMC article. Review. - Oxidoreductases in Glycoprotein Glycosylation, Folding, and ERAD.
Patel C, Saad H, Shenkman M, Lederkremer GZ. Patel C, et al. Cells. 2020 Sep 22;9(9):2138. doi: 10.3390/cells9092138. Cells. 2020. PMID: 32971745 Free PMC article. Review. - A Non-redundant Function of MNS5: A Class I α-1, 2 Mannosidase, in the Regulation of Endoplasmic Reticulum-Associated Degradation of Misfolded Glycoproteins.
Sun X, Guo C, Ali K, Zheng Q, Wei Q, Zhu Y, Wang L, Li G, Li W, Zheng B, Bai Q, Wu G. Sun X, et al. Front Plant Sci. 2022 Apr 19;13:873688. doi: 10.3389/fpls.2022.873688. eCollection 2022. Front Plant Sci. 2022. PMID: 35519817 Free PMC article. - Characterization of the Grp94/OS-9 chaperone-lectin complex.
Seidler PM, Shinsky SA, Hong F, Li Z, Cosgrove MS, Gewirth DT. Seidler PM, et al. J Mol Biol. 2014 Oct 23;426(21):3590-605. doi: 10.1016/j.jmb.2014.08.024. Epub 2014 Sep 3. J Mol Biol. 2014. PMID: 25193139 Free PMC article.
References
- Helenius A., Aebi M. ( 2004) Annu. Rev. Biochem. 73, 1019– 1049 - PubMed
- van Anken E., Braakman I. ( 2005) Crit. Rev. Biochem. Mol. Biol. 40, 191– 228 - PubMed
- Raasi S., Wolf D. H. ( 2007) Semin. Cell Dev. Biol. 18, 780– 791 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases