All-you-can-eat: autophagy in neurodegeneration and neuroprotection - PubMed (original) (raw)
All-you-can-eat: autophagy in neurodegeneration and neuroprotection
Philipp A Jaeger et al. Mol Neurodegener. 2009.
Abstract
Autophagy is the major pathway involved in the degradation of proteins and organelles, cellular remodeling, and survival during nutrient starvation. Autophagosomal dysfunction has been implicated in an increasing number of diseases from cancer to bacterial and viral infections and more recently in neurodegeneration. While a decrease in autophagic activity appears to interfere with protein degradation and possibly organelle turnover, increased autophagy has been shown to facilitate the clearance of aggregation-prone proteins and promote neuronal survival in a number of disease models. On the other hand, too much autophagic activity can be detrimental as well and lead to cell death, suggesting the regulation of autophagy has an important role in cell fate decisions. An increasing number of model systems are now available to study the role of autophagy in the central nervous system and how it might be exploited to treat disease. We will review here the current knowledge of autophagy in the central nervous system and provide an overview of the various models that have been used to study acute and chronic neurodegeneration.
Figures
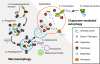
Figure 1
Steps in macroautophagy and chaperone-mediated autophagy (CMA). Macroautophagy: 1.) Nucleation. An unidentified membrane source delivers lipid bi-layers for the formation of the phagophore. In yeast this early structure is termed pre-autophagosomal structure (PAS), its identity in mammalian cells is uncertain. A class III PI3K complex consisting of at least BECN1, PIK3C3, PIK3R4, UVRAG, and AMBRA1 is required for PAS formation and MAP1LC3 is anchored to the membrane via a phosphoethanolamine (PE) anchor (LC3-II). 2.) Expansion. The PAS or a comparable structure in mammals sequesters cytosolic cargo (either specifically via SQSTM1 [p62] or nonspecifically) by invagination, forming a double-membranous vesicle. This stage is also called "isolation membrane". More membrane and LC3-II is being recruited to the developing vacuole. 3.) Maturation. The completed autophagosome undergoes multiple maturation steps and fusion events with multi-vesicular bodies (MVB) or endosomes. The exact nature and sequence of this maturation, and whether these steps are always required is currently unknown. The autophagosomal lumen becomes more acidified during this maturation. 4.) Docking and fusion. During docking and fusion the inner membrane compartment together with its content gets released into the lysosome/autolysosome and is being degraded by lysosomal hydrolases. The components of the outer membrane are available for re-usage. Chaperone-mediated autophagy: 5.) Recognition and binding. The HSC70 chaperone complex (consisting of HSC70, HSP90 and maybe other proteins) recognizes unfolded proteins with the KFERQ sequence and moves them to the lysosome. 6.) Translocation. LAMP2A and a lysosomal form of HSC70 (l-HSC70) translocate the substrate protein across the lysosomal membrane into the lumen for degradation. The autophagy delivered substrates get degraded inside the lysosomes and their macromolecular components are made available to the cell's metabolism via permeases that allow their transport back into the cytosol.
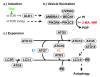
Figure 2
Autophagy pathway in mammals. The formation of autophagosomes appears to follow a pathway conserved across species and most findings made in yeast or other organisms also apply to mammalian autophagy. a.) Autophagy can be induced via mTOR dependent or independent pathways (for more information, see text and Fig. 3) which stimulate the nucleation and expansion of the phagophore/isolation membrane. b.) A multi-protein complex surrounding BECN1 with PI3K activity (mediated by PIK3C3) is important for the formation of the autophagosomal membrane. c.) Two ubiquitin-like modification systems are essential for mammalian autophagy; ATG12 is activated by ATG7 (E1 step), transferred to ATG10 (E2 step), conjugated to ATG5 and subsequently forms a complex with ATG16. This step is necessary early in autophagy for the formation of the phagophore or isolation membrane. MAP1LC3 (LC3) is cleaved by ATG4, activated by ATG7 (E1 step), transferred to ATG3 (E2 step), and conjugated to the phospholipid phosphoethanolamine (PE). This form known as MAP1LC3-II (LC3-II), localizes to the autophagosome membrane and is subsequently degraded in the lysosome. ATG4 cleaves off a C-terminal arginine (R) to expose a glycine residue that is then being linked to PE. Rapamycin (Rap) inhibits mTOR and activates macroautophagy, while 3-methyladenin (3-MA) and wortmannin (WM) inhibit the PI3K activity and de-activate macroautophagy.
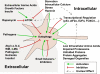
Figure 3
Control of autophagy. Autophagy is a major housekeeping pathway and under the control of many different signaling cascades. Mammalian Target of rapamycin (mTOR) plays a central role in the regulation of autophagic activity as it integrates signaling from different sensors of cellular homeostasis. When mTOR is active in yeast it keeps an important ULK1 binding partner (ATG13) phosphorylated, thus inhibiting the induction of autophagy. While signals indicating abundant nutritional and trophic support activate mTOR (and deactivate autophagy), signals of starvation or other stressors inhibit mTOR (and activate autophagy). Autophagy can be directly stimulated by intracellular debris (such as unfolded proteins and damaged organelles) or by indicators of an overwhelmed ubiquitin-proteasome system (UPS). Also certain pathogens activate autophagy. Autophagy can be directly inhibited by genetic ablation of important Atg genes, inhibitors of the class III PI3K-complex (WM, 3-MA), high nutrient levels, and inositol signaling. More recently screenings of small compound libraries have yielded inducers and inhibitors of autophagy, both mTOR dependent and independent. And last, transcriptional regulators, such as p53, eIF2α, E2F4, or FOXO3 regulate autophagy by controlling the expression levels of many Atg genes. For further details, please refer to the text.
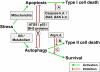
Figure 4
Interaction between autophagy and apoptosis. Cellular stressors can lead to mitochondria outer membrane permeabilization (MOMP) and subsequent cytochrome c release and apoptosis, while nutrient deficiency or ER stress can cause autophagy activation. Under physiological conditions autophagy and apoptosis keep each other inactive through mutual inhibition. A strong apoptotic stimulus (for example DNA damage, death-receptor stimulation, or cytokine deprivation) can drive a cell into apoptotic 'type I' cell death. If apoptosis is inhibited under such conditions (by caspase knockout or Bax/Bak knockout, [A]), autophagy can become activated and result in a delayed 'type II' cell death through degradation of most cytoplasmic cell components and organelles. Under these circumstances the knockdown of autophagy related genes [B] reduces cell death. Autophagy can become activated through ER stress (for example accumulation of misfolded proteins in the ER, intracellular calcium release from the ER) or nutrient deficiency. The cell then ensures survival by enhancing metabolic recycling through autophagy and adapting to the new nutrient conditions. Knockdown of autophagy genes in such a situation leads to an increase in apoptotic 'type I' cell death [C]. The crosstalk between autophagy and apoptosis [D] is mediated via proteolytic processing of ATG5, the transcription factor p53, and the binding and subcellular localization of BCL2 family proteins with BH3 domains. For further details, please refer to the references in the text.
Similar articles
- Autophagy in the central nervous system: implications for neurodegenerative disorders.
Xilouri M, Stefanis L. Xilouri M, et al. CNS Neurol Disord Drug Targets. 2010 Dec;9(6):701-19. doi: 10.2174/187152710793237421. CNS Neurol Disord Drug Targets. 2010. PMID: 20942791 Review. - An Update on Autophagy in Prion Diseases.
López-Pérez Ó, Badiola JJ, Bolea R, Ferrer I, Llorens F, Martín-Burriel I. López-Pérez Ó, et al. Front Bioeng Biotechnol. 2020 Aug 27;8:975. doi: 10.3389/fbioe.2020.00975. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32984276 Free PMC article. Review. - To die or not to die: that is the autophagic question.
Galluzzi L, Vicencio JM, Kepp O, Tasdemir E, Maiuri MC, Kroemer G. Galluzzi L, et al. Curr Mol Med. 2008 Mar;8(2):78-91. doi: 10.2174/156652408783769616. Curr Mol Med. 2008. PMID: 18336289 Review. - Review: autophagy in neurodegeneration: firefighter and/or incendiarist?
Rami A. Rami A. Neuropathol Appl Neurobiol. 2009 Oct;35(5):449-61. doi: 10.1111/j.1365-2990.2009.01034.x. Epub 2009 Jun 25. Neuropathol Appl Neurobiol. 2009. PMID: 19555462 Review. - Selective autophagy in Drosophila.
Nezis IP. Nezis IP. Int J Cell Biol. 2012;2012:146767. doi: 10.1155/2012/146767. Epub 2012 Apr 10. Int J Cell Biol. 2012. PMID: 22567011 Free PMC article.
Cited by
- LC3 Immunostaining in the Inferior Olivary Nuclei of Cats With Niemann-Pick Disease Type C1 Is Associated With Patterned Purkinje Cell Loss.
Gurda BL, Bagel JH, Fisher SJ, Schultz ML, Lieberman AP, Hand P, Vite CH, Swain GP. Gurda BL, et al. J Neuropathol Exp Neurol. 2018 Mar 1;77(3):229-245. doi: 10.1093/jnen/nlx119. J Neuropathol Exp Neurol. 2018. PMID: 29346563 Free PMC article. - The ER retention protein RER1 promotes alpha-synuclein degradation via the proteasome.
Park HJ, Ryu D, Parmar M, Giasson BI, McFarland NR. Park HJ, et al. PLoS One. 2017 Sep 6;12(9):e0184262. doi: 10.1371/journal.pone.0184262. eCollection 2017. PLoS One. 2017. PMID: 28877262 Free PMC article. - Novel synthetic small-molecule activators of AMPK as enhancers of autophagy and amyloid-β peptide degradation.
Vingtdeux V, Chandakkar P, Zhao H, d'Abramo C, Davies P, Marambaud P. Vingtdeux V, et al. FASEB J. 2011 Jan;25(1):219-31. doi: 10.1096/fj.10-167361. Epub 2010 Sep 17. FASEB J. 2011. PMID: 20852062 Free PMC article. - Development and characterization of a new Parkinson's disease model resulting from impaired autophagy.
Ahmed I, Liang Y, Schools S, Dawson VL, Dawson TM, Savitt JM. Ahmed I, et al. J Neurosci. 2012 Nov 14;32(46):16503-9. doi: 10.1523/JNEUROSCI.0209-12.2012. J Neurosci. 2012. PMID: 23152632 Free PMC article. - Links between autophagy and disorders of glycogen metabolism - Perspectives on pathogenesis and possible treatments.
Farah BL, Yen PM, Koeberl DD. Farah BL, et al. Mol Genet Metab. 2020 Jan;129(1):3-12. doi: 10.1016/j.ymgme.2019.11.005. Epub 2019 Nov 21. Mol Genet Metab. 2020. PMID: 31787497 Free PMC article. Review.
References
- Ciechanover A, Brundin P. The ubiquitin proteasome system in neurodegenerative diseases: sometimes the chicken, sometimes the egg. Neuron. 2003;40:427–446. - PubMed
- Rubinsztein DC. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature. 2006;443:780–786. - PubMed
- Klionsky DJ. Autophagy revisited: a conversation with Christian de Duve. Autophagy. 2008;4:740–743. - PubMed
LinkOut - more resources
Full Text Sources