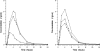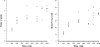Pharmacokinetics of nikkomycin Z after single rising oral doses - PubMed (original) (raw)
Randomized Controlled Trial
. 2009 Jun;53(6):2517-21.
doi: 10.1128/AAC.01609-08. Epub 2009 Apr 6.
Affiliations
- PMID: 19349517
- PMCID: PMC2687243
- DOI: 10.1128/AAC.01609-08
Randomized Controlled Trial
Pharmacokinetics of nikkomycin Z after single rising oral doses
David E Nix et al. Antimicrob Agents Chemother. 2009 Jun.
Abstract
Nikkomycin Z is an antifungal drug that inhibits chitin synthase. This agent is under development as an orphan product for treatment of coccidioidomycosis. Safety and pharmacokinetics of nikkomycin Z were evaluated in healthy male subjects following single, rising oral doses ranging from 250 mg to 2,000 mg. A total of 12 subjects were recruited and divided into two groups. Group 1 (n = 6) received two out of three doses of 250 mg, 1,000 mg, or 1,750 mg and a placebo randomly in place of one of the doses. Group 2 (n = 6) received two out of three doses of 500 mg, 1,500 mg, or 2,000 mg and a placebo in place of one of the doses. Subjects were confined to the study unit overnight prior to dosing, and 12 blood samples were collected over 24 h postdosing while subjects were confined. Subjects returned for additional blood samples and safety evaluations at 48 h and 72 h after each dose. There was a 2-week washout period between doses. Plasma drug concentrations were determined using a validated high-performance liquid chromatography method. Nikkomycin Z was absorbed after oral administration, reaching a maximum concentration in serum of 2.21 microg/ml at 2 h postdose and an area under the concentration-time curve from 0 h to infinity of 11.3 microg x h/ml for the 250-mg dose. Pharmacokinetics appeared linear over the range of 250 to 500 mg; however, relative bioavailability was about 62 to 70% for the 1,000-mg dose and 42 to 47% for doses between 1,500 and 2,000 mg. The mean terminal half-life ranged from 2.1 to 2.5 h and was independent of dose. No serious or dose-related adverse events were observed. This study provides a basis for pharmacokinetic simulations and continued studies of nikkomycin Z administered in multiple doses.
Figures
FIG. 1.
Mean concentration (in micrograms per milliliter) of nikkomycin versus time for the two subject groups (n = 4 per dose level). The left panel includes data for subjects who received doses of 250 mg (○), 1,000 mg (□), and 1,750 mg (Δ) (group 1). The right panel includes data for doses of 500 mg (○), 1,500 mg (□), and 2,000 mg (Δ) (group 2).
FIG. 2.
Dose proportionality of nikkomycin Z based on the _C_max (left) and AUC0-∞ (right). Crossbars represent mean values for each dose level.
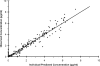
FIG. 3.
Goodness of fit for the selected population pharmacokinetic model. Observed plasma drug concentrations are shown versus the predicted concentrations. The solid line represents the line of identity.
Similar articles
- Modeling nikkomycin Z dosing and pharmacology in murine pulmonary coccidioidomycosis preparatory to phase 2 clinical trials.
Shubitz LF, Trinh HT, Perrill RH, Thompson CM, Hanan NJ, Galgiani JN, Nix DE. Shubitz LF, et al. J Infect Dis. 2014 Jun 15;209(12):1949-54. doi: 10.1093/infdis/jiu029. Epub 2014 Jan 12. J Infect Dis. 2014. PMID: 24421256 Free PMC article. - Pharmacokinetics of dexloxiglumide after administration of single and repeat oral escalating doses in healthy young males.
Persiani S, D'Amato M, Makovec F, Tavares IA, Bishai PM, Rovati LC. Persiani S, et al. Int J Clin Pharmacol Ther. 2002 May;40(5):198-206. doi: 10.5414/cpp40198. Int J Clin Pharmacol Ther. 2002. PMID: 12051571 Clinical Trial. - Double-blind evaluation of the safety and pharmacokinetics of multiple oral once-daily 750-milligram and 1-gram doses of levofloxacin in healthy volunteers.
Chien SC, Wong FA, Fowler CL, Callery-D'Amico SV, Williams RR, Nayak R, Chow AT. Chien SC, et al. Antimicrob Agents Chemother. 1998 Apr;42(4):885-8. doi: 10.1128/AAC.42.4.885. Antimicrob Agents Chemother. 1998. PMID: 9559801 Free PMC article. Clinical Trial. - Evaluation of nikkomycin Z with frequent oral administration in an experimental model of central nervous system coccidioidomycosis.
Wiederhold NP, Najvar LK, Jaramillo R, Olivo M, Larwood DJ, Patterson TF. Wiederhold NP, et al. Microbiol Spectr. 2024 Oct 3;12(10):e0135624. doi: 10.1128/spectrum.01356-24. Epub 2024 Aug 20. Microbiol Spectr. 2024. PMID: 39162491 Free PMC article. - Pharmacokinetics and safety of voriconazole following intravenous- to oral-dose escalation regimens.
Purkins L, Wood N, Ghahramani P, Greenhalgh K, Allen MJ, Kleinermans D. Purkins L, et al. Antimicrob Agents Chemother. 2002 Aug;46(8):2546-53. doi: 10.1128/AAC.46.8.2546-2553.2002. Antimicrob Agents Chemother. 2002. PMID: 12121931 Free PMC article. Clinical Trial.
Cited by
- An insight into the antifungal pipeline: selected new molecules and beyond.
Ostrosky-Zeichner L, Casadevall A, Galgiani JN, Odds FC, Rex JH. Ostrosky-Zeichner L, et al. Nat Rev Drug Discov. 2010 Sep;9(9):719-27. doi: 10.1038/nrd3074. Epub 2010 Aug 20. Nat Rev Drug Discov. 2010. PMID: 20725094 Review. - Novel Promising Antifungal Target Proteins for Conquering Invasive Fungal Infections.
Zhen C, Lu H, Jiang Y. Zhen C, et al. Front Microbiol. 2022 Jun 16;13:911322. doi: 10.3389/fmicb.2022.911322. eCollection 2022. Front Microbiol. 2022. PMID: 35783432 Free PMC article. Review. - Natural Antimicrobial Peptides as Inspiration for Design of a New Generation Antifungal Compounds.
Bondaryk M, Staniszewska M, Zielińska P, Urbańczyk-Lipkowska Z. Bondaryk M, et al. J Fungi (Basel). 2017 Aug 26;3(3):46. doi: 10.3390/jof3030046. J Fungi (Basel). 2017. PMID: 29371563 Free PMC article. Review. - Nature's combinatorial biosynthesis and recently engineered production of nucleoside antibiotics in Streptomyces.
Chen S, Kinney WA, Van Lanen S. Chen S, et al. World J Microbiol Biotechnol. 2017 Apr;33(4):66. doi: 10.1007/s11274-017-2233-6. Epub 2017 Mar 4. World J Microbiol Biotechnol. 2017. PMID: 28260195 Review. - Conserved Mechanism of 2'-Phosphorylation-Aided Amide Ligation in Peptidyl Nucleoside Biosynthesis.
Draelos MM, Thanapipatsiri A, Yokoyama K. Draelos MM, et al. Biochemistry. 2021 Jul 20;60(28):2231-2235. doi: 10.1021/acs.biochem.1c00327. Epub 2021 Jul 9. Biochemistry. 2021. PMID: 34242001 Free PMC article.
References
- Galgiani, J. N., N. M. Ampel, J. E. Blair, A. Catanzaro, R. H. Johnson, D. A. Stevens, and P. L. Williams. 2005. Coccidioidomycosis. Clin. Infect. Dis. 41:1217-1223. - PubMed
- Goldberg, J., P. Connolly, C. Schnizlein-Bick, M. Durkin, S. Kohler, M. Smedema, E. Brizendine, R. Hector, and J. Wheat. 2000. Comparison of nikkomycin Z with amphotericin B and itraconazole for treatment of histoplasmosis in a murine model. Antimicrob. Agents Chemother. 44:1624-1629. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources