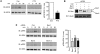beta-Arrestin1 mediates nicotinic acid-induced flushing, but not its antilipolytic effect, in mice - PubMed (original) (raw)
. 2009 May;119(5):1312-21.
doi: 10.1172/JCI36806. Epub 2009 Apr 6.
Affiliations
- PMID: 19349687
- PMCID: PMC2673863
- DOI: 10.1172/JCI36806
beta-Arrestin1 mediates nicotinic acid-induced flushing, but not its antilipolytic effect, in mice
Robert W Walters et al. J Clin Invest. 2009 May.
Abstract
Nicotinic acid is one of the most effective agents for both lowering triglycerides and raising HDL. However, the side effect of cutaneous flushing severely limits patient compliance. As nicotinic acid stimulates the GPCR GPR109A and Gi/Go proteins, here we dissected the roles of G proteins and the adaptor proteins, beta-arrestins, in nicotinic acid-induced signaling and physiological responses. In a human cell line-based signaling assay, nicotinic acid stimulation led to pertussis toxin-sensitive lowering of cAMP, recruitment of beta-arrestins to the cell membrane, an activating conformational change in beta-arrestin, and beta-arrestin-dependent signaling to ERK MAPK. In addition, we found that nicotinic acid promoted the binding of beta-arrestin1 to activated cytosolic phospholipase A2 as well as beta-arrestin1-dependent activation of cytosolic phospholipase A2 and release of arachidonate, the precursor of prostaglandin D2 and the vasodilator responsible for the flushing response. Moreover, beta-arrestin1-null mice displayed reduced cutaneous flushing in response to nicotinic acid, although the improvement in serum free fatty acid levels was similar to that observed in wild-type mice. These data suggest that the adverse side effect of cutaneous flushing is mediated by beta-arrestin1, but lowering of serum free fatty acid levels is not. Furthermore, G protein-biased ligands that activate GPR109A in a beta-arrestin-independent fashion may represent an improved therapeutic option for the treatment of dyslipidemia.
Figures
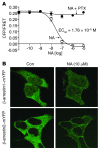
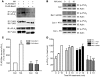
Figure 1. Nicotinic acid–induced decrease in cAMP, and increase in β-arrestin membrane recruitment, in GPR109A-expressing HEK-293 cells.
Cells also expressing the ICUE2 biosensor (see Methods) were treated with forskolin and increasing concentrations of nicotinic acid (NA). (A) Nicotinic acid (open circles) decreased cAMP in a dose-dependent fashion, and this response was inhibited by pertussis toxin (PTX; filled circles). Data are mean ± SEM of 3 independent experiments. CFP, cyan fluorescent protein; FRET, fluorescence resonance energy transfer. (B) Cells were transfected with either β-arrestin1–mYFP or β-arrestin2–mYFP. Both β-arrestin isoforms resided in the cytosol prior to nicotinic acid stimulation (control; Con), and translocated to bind GPR109A in the membrane in response to treatment with 10 μM nicotinic acid. No translocation was noted in cells lacking GPR109A (not shown). Images are representative of 4 independent experiments. Original magnification, ×100.
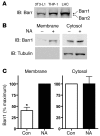
Figure 2. Adipocytes, macrophages, and Langerhans cells express β-arrestins, and β-arrestin1 is recruited to the cell membrane with stimulation of GPR109A in Langerhans cells.
(A) Cell lysates from differentiated 3T3-L1 adipocytes, differentiated THP-1 macrophages, and Langerhans cells (LHC) expressed both β-arrestin1 (Barr1) and β-arrestin2 (Barr2). (B) After stimulation with 10 μM nicotinic acid for 10 minutes, Langerhans cells were harvested, and membranes were separated from the cytosol, as demonstrated by presence of tubulin only in the cytosolic fractions. Increased β-arrestin1 was detected in the membranes after nicotinic acid stimulation, in contrast to control-treated samples. (C) Recruitment of β-arrestin1 to the membrane after nicotinic acid stimulation. *P = 0.0014 versus nicotinic acid. Data are mean ± SEM of 3 independent experiments.
Figure 3. Conformational change in β-arrestin2 upon activation of GPR109A.
(A) Dose dependency of the conformational changes in β-arrestin2 upon stimulation of GPR109A by nicotinic acid. Filled squares, cells expressing Luc–β-arr–YFP and empty vector; open squares, cells expressing Luc–β-arr–YFP and GPR109A. (B) Kinetics of nicotinic acid–induced conformational change in β-arrestin. Data are mean ± SEM of 4 independent experiments.
Figure 4. Nicotinic acid–stimulated phosphorylation of ERK.
(A) GPR109A-expressing HEK-293 cells were stimulated with 200 μM nicotinic acid, and cell lysates were analyzed for phosphorylated ERK (pERK) at varying times. Agonist stimulated activation of ERK in the presence or absence of pertussis toxin. tERK, total ERK. *P = 0.027 versus control. (B) Expression of β-arrestin decreased after siRNA treatment. (C) Agonist stimulated ERK activation in the presence of control, β-arrestin1, β-arrestin2, or β-arrestin1 and β-arrestin2 siRNA. Graph shows phosphorylation of ERK 10 minutes after stimulation. **P < 0.05 versus control. Data are mean ± SEM of 3–6 independent experiments.
Figure 5. Nicotinic acid–induced binding of β-arrestin to cPLA2 and phosphorylated cPLA2.
(A and B) GPR109A-expressing HEK-293 cells were stimulated with 10 μM nicotinic acid or control for 10 minutes. Nicotinic acid stimulation increased binding of β-arrestin to cPLA2 (A) and phosphorylated cPLA2 (p-cPLA2) (B). Arrow indicates phosphorylated cPLA2 band. Equivalent amounts of cPLA2 were present in each whole cell lysate (WCL). Equal amounts of β-arrestin were immunoprecipitated in control and nicotinic acid–treated samples. Moreover, β-arrestin was not immunoprecipitated with preimmune serum (not shown). (C and D) Binding of β-arrestin to cPLA2 (C) and phosphorylated cPLA2 (D). *P = 0.0075, **P = 0.015 versus control. Data are mean ± SEM of 5 independent experiments.
Figure 6. Role of β-arrestin1 in binding and activation of cPLA2.
(A) GPR109A-expressing HEK-293 cells were transfected with FLAG–β-arrestin1 or FLAG–β-arrestin2. Nicotinic acid stimulation increased binding of cPLA2 to FLAG–β-arrestin1, but not FLAG–β-arrestin2. (B) Equivalent amounts of cPLA2 and FLAG–β-arrestins were present in each whole cell lysate. Equal amounts of FLAG–β-arrestin were immunoprecipitated in control and nicotinic acid–treated samples. GPR109A-expressing HEK-293 cells were stimulated with 200 μM nicotinic acid, and cell lysates were analyzed for phosphorylated cPLA2 at varying times. Agonist-stimulated activation of cPLA2 in the presence of control siRNA, β-arrestin1 siRNA, or control siRNA plus either pertussis toxin or PD98059 (PD). (C) Binding of FLAG–β-arrestin to cPLA2. *P = 0.0004 versus respective control. (D) Activation or phosphorylation of cPLA2 in siRNA-treated cells. **P = 0.0085 versus respective 10-minute value; ***P = 0.0047 versus respective 0-minute value. Data are mean ± SEM of 3 independent experiments.
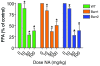
Figure 7. Nicotinic acid induces antilipolysis in wild-type and β-arrestin–deficient mice.
Nicotinic acid decreased FFA levels in wild-type C57BL/6 mice as well as mice deficient in β-arrestin1 or β-arrestin2. Nonesterified FFAs were measured in mice given i.p. injections of either vehicle alone or nicotinic acid at a dose of 10, 50, or 100 mg/kg. FFA levels are expressed as a percent of vehicle-treated control animals for each genotype. *P < 0.0001 comparing the interaction of dose. The change in FFAs after nicotinic acid stimulation was not significantly different between wild-type, β-arrestin1–deficient, and β-arrestin2–deficient mice. Data are mean ± SEM in control or nicotinic acid–treated animals (n = 4–10 per condition).
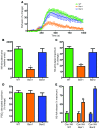
Figure 8. Nicotinic acid–induced cutaneous flushing and activity of cPLA2 is attenuated in β-arrestin1–deficient mice.
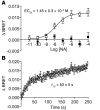
(A) Perfusion of the ventral ear in wild-type, β-arrestin1–deficient, or β-arrestin2–deficient mice was measured with laser Doppler. Baseline perfusion was measured for 150 seconds, then mice were given i.p. injections of 100 mg/kg nicotinic acid. Data are mean ± SEM for change in perfusion as a function of time. (B) Total response to nicotinic acid, plotted as mean ± SEM area under the curve. (C) Maximum response to nicotinic acid, plotted as mean ± SEM. *P < 0.0001 versus wild type. (D) Maximum response to PGD2, plotted as mean ± SEM (n = 15–25 per condition). (E) Nicotinic acid–stimulated release of eicosanoids was measured in peritoneal macrophages. Thioglycollate-elicited peritoneal macrophages were loaded with H3-arachidonic acid (H3-AA) for 24 hours, and then rinsed to remove arachidonic acid not incorporated into cell membrane lipids. Macrophages were stimulated for 10 minutes with 200 μM nicotinic acid, and radioactivity released into the media was measured. Data are mean ± SEM (n = 4 per condition) for change in radioactivity, plotted as a percentage of the maximum response. **P = 0.0001 versus nicotinic acid–treated wild type.
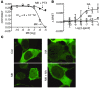
Figure 9. MK-0345–induced G protein signaling, β-arrestin conformational changes, and recruitment.
(A) Cells expressing GPR109A and the ICUE2 biosensor were treated with forskolin and MK-0354 (MK). MK-0354 (open circles) decreased cAMP in a dose-dependent fashion, and this response was inhibited by pertussis toxin (filled circles). (B) Cells expressing GPR109A and the BRET reporter Luc–β-arr–YFP were treated with nicotinic acid (open squares) or MK-0354 (open circles). MK-0354 failed to induce conformational changes in β-arrestin2. Data are mean ± SEM of 3 independent experiments. (C) Cells expressing GPR109A β-arrestin1–mYFP were treated with nicotinic acid, MK-0354, or both. Prior to nicotinic acid stimulation, β-arrestin1 resided in the cytosol; it translocated to bind GPR109A in the membrane in response to 10 μM nicotinic acid. No translocation was noted in cells stimulated with 200 μM MK-0354 or in cells treated with 10 μM nicotinic acid in the presence of 200 μM MK-0354. Images are representative of 4 independent experiments. Original magnification, ×100.
Similar articles
- GPR109A (PUMA-G/HM74A) mediates nicotinic acid-induced flushing.
Benyó Z, Gille A, Kero J, Csiky M, Suchánková MC, Nüsing RM, Moers A, Pfeffer K, Offermanns S. Benyó Z, et al. J Clin Invest. 2005 Dec;115(12):3634-40. doi: 10.1172/JCI23626. J Clin Invest. 2005. PMID: 16322797 Free PMC article. - Discovery of 4-(phenyl)thio-1H-pyrazole derivatives as agonists of GPR109A, a high affinity niacin receptor.
Kim HY, Jadhav VB, Jeong DY, Park WK, Song JH, Lee S, Cho H. Kim HY, et al. Arch Pharm Res. 2015 Jun;38(6):1019-32. doi: 10.1007/s12272-015-0560-4. Epub 2015 Jan 20. Arch Pharm Res. 2015. PMID: 25599616 - Langerhans cells release prostaglandin D2 in response to nicotinic acid.
Maciejewski-Lenoir D, Richman JG, Hakak Y, Gaidarov I, Behan DP, Connolly DT. Maciejewski-Lenoir D, et al. J Invest Dermatol. 2006 Dec;126(12):2637-46. doi: 10.1038/sj.jid.5700586. Epub 2006 Sep 28. J Invest Dermatol. 2006. PMID: 17008871 - Nicotinic acid (niacin): new lipid-independent mechanisms of action and therapeutic potentials.
Lukasova M, Hanson J, Tunaru S, Offermanns S. Lukasova M, et al. Trends Pharmacol Sci. 2011 Dec;32(12):700-7. doi: 10.1016/j.tips.2011.08.002. Epub 2011 Sep 22. Trends Pharmacol Sci. 2011. PMID: 21944259 Review. - Future of GPR109A agonists in the treatment of dyslipidaemia.
Wanders D, Judd RL. Wanders D, et al. Diabetes Obes Metab. 2011 Aug;13(8):685-91. doi: 10.1111/j.1463-1326.2011.01400.x. Diabetes Obes Metab. 2011. PMID: 21418500 Review.
Cited by
- Signalling bias in new drug discovery: detection, quantification and therapeutic impact.
Kenakin T, Christopoulos A. Kenakin T, et al. Nat Rev Drug Discov. 2013 Mar;12(3):205-16. doi: 10.1038/nrd3954. Epub 2012 Feb 15. Nat Rev Drug Discov. 2013. PMID: 23411724 Review. - Understanding G Protein Selectivity of Muscarinic Acetylcholine Receptors Using Computational Methods.
Santiago LJ, Abrol R. Santiago LJ, et al. Int J Mol Sci. 2019 Oct 24;20(21):5290. doi: 10.3390/ijms20215290. Int J Mol Sci. 2019. PMID: 31653051 Free PMC article. - Oligomeric Receptor Complexes and Their Allosteric Receptor-Receptor Interactions in the Plasma Membrane Represent a New Biological Principle for Integration of Signals in the CNS.
Borroto-Escuela DO, Fuxe K. Borroto-Escuela DO, et al. Front Mol Neurosci. 2019 Sep 25;12:230. doi: 10.3389/fnmol.2019.00230. eCollection 2019. Front Mol Neurosci. 2019. PMID: 31607863 Free PMC article. - Emerging paradigms of β-arrestin-dependent seven transmembrane receptor signaling.
Shukla AK, Xiao K, Lefkowitz RJ. Shukla AK, et al. Trends Biochem Sci. 2011 Sep;36(9):457-69. doi: 10.1016/j.tibs.2011.06.003. Epub 2011 Jul 20. Trends Biochem Sci. 2011. PMID: 21764321 Free PMC article. Review. - The beta-arrestin pathway-selective type 1A angiotensin receptor (AT1A) agonist [Sar1,Ile4,Ile8]angiotensin II regulates a robust G protein-independent signaling network.
Kendall RT, Strungs EG, Rachidi SM, Lee MH, El-Shewy HM, Luttrell DK, Janech MG, Luttrell LM. Kendall RT, et al. J Biol Chem. 2011 Jun 3;286(22):19880-91. doi: 10.1074/jbc.M111.233080. Epub 2011 Apr 18. J Biol Chem. 2011. PMID: 21502318 Free PMC article.
References
- Canner P.L., et al. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J. Am. Coll. Cardiol. 1986;8:1245–1255. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK58398/DK/NIDDK NIH HHS/United States
- P01 DK058398/DK/NIDDK NIH HHS/United States
- HL16037/HL/NHLBI NIH HHS/United States
- R01 HL016037/HL/NHLBI NIH HHS/United States
- R01 HL070631/HL/NHLBI NIH HHS/United States
- HL70631/HL/NHLBI NIH HHS/United States
- 5T32 A1007217-25/PHS HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous