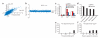A molecular barcoded yeast ORF library enables mode-of-action analysis of bioactive compounds - PubMed (original) (raw)
doi: 10.1038/nbt.1534. Epub 2009 Apr 6.
Leslie Magtanong # 1 2, Sarah L Barker # 1 2, David Gresham 3, Shinichi Nishimura 4 5, Paramasivam Natarajan 6, Judice L Y Koh 1 2, Justin Porter 7, Christopher A Gray 7, Raymond J Andersen 7, Guri Giaever 1 2 8, Corey Nislow 1 2, Brenda Andrews 1 2, David Botstein 3, Todd R Graham 6, Minoru Yoshida 4, Charles Boone 1 2 4
Affiliations
- PMID: 19349972
- PMCID: PMC3856559
- DOI: 10.1038/nbt.1534
A molecular barcoded yeast ORF library enables mode-of-action analysis of bioactive compounds
Cheuk Hei Ho et al. Nat Biotechnol. 2009 Apr.
Abstract
We present a yeast chemical-genomics approach designed to identify genes that when mutated confer drug resistance, thereby providing insight about the modes of action of compounds. We developed a molecular barcoded yeast open reading frame (MoBY-ORF) library in which each gene, controlled by its native promoter and terminator, is cloned into a centromere-based vector along with two unique oligonucleotide barcodes. The MoBY-ORF resource has numerous genetic and chemical-genetic applications, but here we focus on cloning wild-type versions of mutant drug-resistance genes using a complementation strategy and on simultaneously assaying the fitness of all transformants with barcode microarrays. The complementation cloning was validated by mutation detection using whole-genome yeast tiling microarrays, which identified unique polymorphisms associated with a drug-resistant mutant. We used the MoBY-ORF library to identify the genetic basis of several drug-resistant mutants and in this analysis discovered a new class of sterol-binding compounds.
Figures
Figure 1
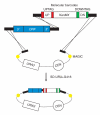
Construction of the MoBY-ORF library by homologous recombination in yeast. Yeast cells are co-transformed with a PCR product encoding an ORF, a KanMX cassette, which confers G418/kanamycin resistance, and an _Xho_I-linearized vector that carries a selectable marker (URA3) and a yeast centromere sequence (CEN). Transformants are grown on synthetic medium that selects for recombinant plasmids. The KanMX cassette is flanked by two different molecular barcodes, labeled UPTAG and DOWNTAG, each of which are flanked by common primer sites, indicated in red and green. The KanMX barcode cassette for each gene was amplified from the corresponding deletion mutant for that gene. The vector backbone was designed to be compatible with the MAGIC system for manipulating plasmid inserts by homologous recombination in Escherichia coli. Filled yellow circles represent MAGIC recombination sites flanking the MoBY-ORF inserts.
Figure 2
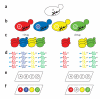
Identifying a recessive spontaneous drug-resistant mutant by MoBY-ORF complementation cloning. (a) Isolate a drug-resistant mutant. A chromosomal mutation in gene B leads to a drug-resistant allele (designated b). (b) Transform mutant with the MoBY-ORF library. In the example, plasmids carry wild-type copies of genes A, B, C and D. (c) Grow the pool of transformants in the absence and presence of a drug. The wild-type B allele complements the drug-resistant allele, causing the transformant that received B to be depleted from the pool with drug. (d) Extract plasmid DNA, PCR amplify and fluorescently label the barcode sequences. (e) Hybridize labeled barcode DNA to an Affymetrix TAG4 microarray. (f) Identify the transformant most depleted in the drug treated pool, in this case B.
Figure 3
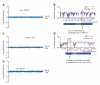
Mapping a drug-resistant mutant by MoBY-ORF complementation cloning and yeast tiling microarrays. (a) Barcode depletion plot showing the relative levels of all MoBY-ORF plasmids in library-transformed, cycloheximide-resistant mutants (cycR) grown in cyclohexamide (1 μg/ml). Each point represents the relative abundance of a single plasmid in the untreated compared to drug-treated growth cultures (as measured by the _y_-axis ratio). An average value of the ratio of two independent experiments is shown. RPL28 (YGL103W) was the ORF most depleted in the drug-treated pool. (b) Polymorphism prediction at the RPL28 locus in cycR mutant by whole-genome YTM analysis. Red and blue lines trace the log of the predictive signal (the likelihood ratio) for the presence of a SNP within the RPL28 locus on chromosome VII. Direct sequencing identified a single-nucleotide substitution at nucleotide 623 of RPL28. (c) Barcode depletion plot for the rapamycin-resistant (rapR) mutant grown in the presence of rapamycin (5 μg/ml). FPR (YNL135C) was the most depleted ORF. (d) Polymorphism prediction at the FPR1 locus in rapR by whole-genome YTM. Direct sequencing identified a 29 base-pair deletion from nucleotide 282 to 310 of FPR1. (e) Barcode depletion plot for the sordarin-resistant (sorR) mutant grown in the presence of sordarin (50 μg/ml). EFT2 (YDR385W) was the most depleted ORF.
Figure 4
MOA analysis of theopalauamide and stichloroside. (a) Correlation plot of chemical-genetic profiles of theopalauamide and stichloroside (correlation coefficient (R) = 0.859). Points on the plot correspond to the log2 ratio of control/drug-treated signal for each strain of ~5,000 viable haploid yeast deletion mutants (data are from ref. 9). (b) Barcode depletion plot for the theopalauamide- and stichloroside-resistant (theoR) mutant grown in medium containing theopalauamide (2 μg/ml). MVD1 was the most depleted ORF. (c) Genetic evidence that theopalauamide and stichloroside do not inhibit Mvd1p. Growth after 16 h at 30 °C of a temperature-sensitive mutant of MVD1 (mvd1-ts) and wild-type (WT) control in cultures containing theopalauamide (2.5 μg/ml), stichloroside (5 μg/ml), or ketoconazole (10 μg/ml). Bars show the means of three independent experiments. Error bars indidate s.d. (d) Ergosterol rescues the toxicity of theopalauamide and stichloroside. Growth after 16 h at 30 °C of WT cells in the presence of ergosterol (100 μg/ml) and either amphotericin B (10 μg/ml), theopalauamide (2 μg/ml), stichloroside (5 μg/ml), or ketoconazole (10 μg/ml). Number of replicates and error bars are as in c. (e,f) Theopalauamide permeabilizes liposomes containing ergosterol. Leakage of calcein, a fluorescent marker, from phosphatidylcholine liposomes containing ergosterol (40% in e, or a range of concentrations in f) after exposure to theopalauamide (a range of concentrations in e, or 10 μg/ml in f). Leakage was quantified relative to 100% calcein release from liposomes exposed to Triton-X 100. Bars show the averages of the two highly correlated replicates (Supplementary Fig. 6a,b).
Figure 5
Theopalauamide and theonellamide represent a novel class of sterol-binding compound. (a) Amphotericin and nystatin belong to the polyene class of sterol-binding compound. Stichloroside belongs the saponin class, which includes α-tomatine. Theopalauamide and theonellamide A represent a novel class of sterol-binding compound based on their unusual bicyclic structure bridged by a histidinoalanine residue. (b) In vitro binding of fluorescently labeled theonellamide A (theonellamide-AMCA) to ergosterol. Bars show the means of three independent experiments. PC, phosphatidylcholine; PE, phosphatidylethanol; PS, phosphatidylserine; SM, sphingomyelin. Error bars indicate s.d. (c) The theopalauamide/stichloroside-resistant (theoR) mutant is resistant to theonellamide (12.5 μg/ml after 16 h of growth at 30 °C). Bars show the means of three independent experiments. Error bars indicate s.d. (d) Localization of theonellamide visualized by fluorescent microscopy. Cells were grown to mid-log phase.
Comment in
- A whale of a library.
Xu D, Roemer T. Xu D, et al. Nat Biotechnol. 2009 Apr;27(4):342-4. doi: 10.1038/nbt0409-342. Nat Biotechnol. 2009. PMID: 19352370 No abstract available.
Similar articles
- A whale of a library.
Xu D, Roemer T. Xu D, et al. Nat Biotechnol. 2009 Apr;27(4):342-4. doi: 10.1038/nbt0409-342. Nat Biotechnol. 2009. PMID: 19352370 No abstract available. - The full-ORF clone resource of the German cDNA Consortium.
Bechtel S, Rosenfelder H, Duda A, Schmidt CP, Ernst U, Wellenreuther R, Mehrle A, Schuster C, Bahr A, Blöcker H, Heubner D, Hoerlein A, Michel G, Wedler H, Köhrer K, Ottenwälder B, Poustka A, Wiemann S, Schupp I. Bechtel S, et al. BMC Genomics. 2007 Oct 31;8:399. doi: 10.1186/1471-2164-8-399. BMC Genomics. 2007. PMID: 17974005 Free PMC article. - Phage display of cDNA libraries: enrichment of cDNA expression using open reading frame selection.
Faix PH, Burg MA, Gonzales M, Ravey EP, Baird A, Larocca D. Faix PH, et al. Biotechniques. 2004 Jun;36(6):1018-22, 1024, 1026-9. doi: 10.2144/04366RR03. Biotechniques. 2004. PMID: 15211753 - Many paths to many clones: a comparative look at high-throughput cloning methods.
Marsischky G, LaBaer J. Marsischky G, et al. Genome Res. 2004 Oct;14(10B):2020-8. doi: 10.1101/gr.2528804. Genome Res. 2004. PMID: 15489321 Review. - High-throughput cloning and expression library creation for functional proteomics.
Festa F, Steel J, Bian X, Labaer J. Festa F, et al. Proteomics. 2013 May;13(9):1381-99. doi: 10.1002/pmic.201200456. Epub 2013 Apr 5. Proteomics. 2013. PMID: 23457047 Free PMC article. Review.
Cited by
- An integrated approach for identification and target validation of antifungal compounds active against Erg11p.
Hoepfner D, Karkare S, Helliwell SB, Pfeifer M, Trunzer M, De Bonnechose S, Zimmerlin A, Tao J, Richie D, Hofmann A, Reinker S, Frederiksen M, Movva NR, Porter JA, Ryder NS, Parker CN. Hoepfner D, et al. Antimicrob Agents Chemother. 2012 Aug;56(8):4233-40. doi: 10.1128/AAC.06332-11. Epub 2012 May 21. Antimicrob Agents Chemother. 2012. PMID: 22615293 Free PMC article. - Defining principles of combination drug mechanisms of action.
Pritchard JR, Bruno PM, Gilbert LA, Capron KL, Lauffenburger DA, Hemann MT. Pritchard JR, et al. Proc Natl Acad Sci U S A. 2013 Jan 8;110(2):E170-9. doi: 10.1073/pnas.1210419110. Epub 2012 Dec 18. Proc Natl Acad Sci U S A. 2013. PMID: 23251029 Free PMC article. - Sources of Error in Mammalian Genetic Screens.
Sack LM, Davoli T, Xu Q, Li MZ, Elledge SJ. Sack LM, et al. G3 (Bethesda). 2016 Sep 8;6(9):2781-90. doi: 10.1534/g3.116.030973. G3 (Bethesda). 2016. PMID: 27402361 Free PMC article. - Adaptation of the yeast gene knockout collection is near-perfectly predicted by fitness and diminishing return epistasis.
Persson K, Stenberg S, Tamás MJ, Warringer J. Persson K, et al. G3 (Bethesda). 2022 Nov 4;12(11):jkac240. doi: 10.1093/g3journal/jkac240. G3 (Bethesda). 2022. PMID: 36083011 Free PMC article. - Exploring Quantitative Yeast Phenomics with Single-Cell Analysis of DNA Damage Foci.
Styles EB, Founk KJ, Zamparo LA, Sing TL, Altintas D, Ribeyre C, Ribaud V, Rougemont J, Mayhew D, Costanzo M, Usaj M, Verster AJ, Koch EN, Novarina D, Graf M, Luke B, Muzi-Falconi M, Myers CL, Mitra RD, Shore D, Brown GW, Zhang Z, Boone C, Andrews BJ. Styles EB, et al. Cell Syst. 2016 Sep 28;3(3):264-277.e10. doi: 10.1016/j.cels.2016.08.008. Epub 2016 Sep 8. Cell Syst. 2016. PMID: 27617677 Free PMC article.
References
- Giaever G, et al. Genomic profiling of drug sensitivities via induced haploinsufficiency. Nat. Genet. 1999;21:278–283. - PubMed
- Hoon S, et al. An integrated platform of genomic assays reveals small-molecule bioactivities. Nat. Chem. Biol. 2008;4:498–506. - PubMed
- Butcher RA, et al. Microarray-based method for monitoring yeast overexpression strains reveals small-molecule targets in TOR pathway. Nat. Chem. Biol. 2006;2:103–109. - PubMed
- Luesch H, et al. A genome-wide overexpression screen in yeast for small-molecule target identification. Chem. Biol. 2005;12:55–63. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 GM046406/GM/NIGMS NIH HHS/United States
- R37 GM046406/GM/NIGMS NIH HHS/United States
- R01 GM107466/GM/NIGMS NIH HHS/United States
- GM62637/GM/NIGMS NIH HHS/United States
- P50 GM071508/GM/NIGMS NIH HHS/United States
- GM071508/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases