The endogenous inhibitor of Akt, CTMP, is critical to ischemia-induced neuronal death - PubMed (original) (raw)
The endogenous inhibitor of Akt, CTMP, is critical to ischemia-induced neuronal death
Takahiro Miyawaki et al. Nat Neurosci. 2009 May.
Abstract
Dysregulation of Akt signaling is important in a broad range of diseases that includes cancer, diabetes and heart disease. The role of Akt signaling in brain disorders is less clear. We found that global ischemia in intact rats triggered expression and activation of the Akt inhibitor CTMP (carboxyl-terminal modulator protein) in vulnerable hippocampal neurons and that CTMP bound and extinguished Akt activity and was essential to ischemia-induced neuronal death. Although ischemia induced a marked phosphorylation and nuclear translocation of Akt, phosphorylated Akt was not active in post-ischemic neurons, as assessed by kinase assays and phosphorylation of the downstream targets GSK-3beta and FOXO3A. RNA interference-mediated depletion of CTMP in a clinically relevant model of stroke restored Akt activity and rescued hippocampal neurons. Our results indicate that CTMP is important in the neurodegeneration that is associated with stroke and identify CTMP as a therapeutic target for the amelioration of hippocampal injury and cognitive deficits.
Figures
Figure 1
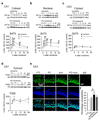
Global ischemia promotes marked phosphorylation and nuclear translocation of the pro-survival kinase Akt in CA1 neurons destined to die. (a) Western blot for p-Akt at Ser 473 in the cytosol. n = 7 animals per treatment group. (b) Western blot for p-Akt at Ser 473 in the nucleus. n = 4 animals per group. (c) Western blot for p-Akt at Ser 473 in the cytosol in CA3. n = 4 animals per group. Protein samples isolated from animals subjected to sham operation, global ischemia (Ischemia), preconditioning (PC), preconditioning followed by global ischemia. Control animals (denoted as 0 h after ischemia) were sacrificed at 12 h after sham operation; preconditioned animals not subjected to ischemia (denoted as 0 h preconditioning) were sacrificed 48 h after preconditioning. Ischemia and preconditioning+ischemia animals were sacrificed 1, 3, 6, 12, 24 h after reperfusion. (d) Western blot for p-Akt at Thr 308 in the cytosol. n = 5 animals per group. (e) Double-labeling of p-Akt (green) and DAPI (blue) in the CA1 at 1 hr after the last surgery. IgG labeling of control tissue reveals little or no background signal. (f)Quantification of colocalization (p-Akt and DAPI). n = 4–5 animals per group. Scale bar = 50 µm. Error bars represent means ± SEM. Significance of experimental vs. control animals is denoted as (*); preconditioning+ischemia vs. Ischemia is denoted as (# ). * /# P < 0.05, ** /## P < 0.01.
Figure 2
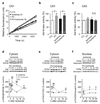
Preconditioning but not ischemia promotes Akt kinase activity and phosphorylation of Akt targets. (a) Akt activity plots of relative fluorescence units vs. time. Experimental animals were sacrificed 3 h after reperfusion. (b) Mean Akt kinase activity in CA1. n = 4 animals per group. (c) Mean Akt kinase activity in CA3. n = 4 animals per group. (d) Western blot for p-GSK-3β at Ser 9 in the cytosol. n = 4–6 animals per group. (e) Western blot for p-FOXO3A at Ser 256 in the cytosol. n = 4–6 animals per group. (f) Western blot for p-FOXO3A at Ser 256 in the nucleus. n = 4–6 animals per group. Error bars represent means ± SEM. Significance is as described in the legend to Fig. 1.
Figure 3
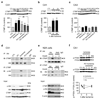
Ischemia promotes CTMP expression and Akt-CTMP assembly in CA1. (a—c) Westerns of CTMP in CA1 (a,b) and CA3 (c) of control, ischemia, preconditioning (PC) and preconditioning+ischemia animals at times after last surgery; CA1, n = 5–7; CA3, n = 4 animals per group and time point. (d) Ischemia promotes and preconditioning attenuates Akt-CTMP assembly in CA1. Upper, co-immunoprecipitation (IP) with anti-Akt and immunoblot (IB) for CTMP. Middle, co-immunoprecipitation with CTMP and immunoblot for p-Akt and Akt. Lower, Input. n = 5–7 animals per group; P < 0.01 vs. control and vs. preconditioning+ischemia). Input shows equal Akt in all groups. (e) Akt-CTMP assembly requires Akt phosphorylation. Upper, co-immunoprecipitation with anti-Akt and immunoblot for CTMP. Middle, co-immunoprecipitation with anti-CTMP and immunoblot for Akt. Lower, Input. N2A cells expressing wild-type (left) or mutant (nonphosphorylatable) Akt(T308A/S473A) (right) under basal (−) or insulin-stimulated (+) conditions were processed for co-immunoprecipitation. In cells expressing WT Akt, Akt/CTMP association is modest under basal (lane 1) and marked under stimulated (lane 2) conditions. In cells expressing mutant Akt(T308A/S473A), Akt/CTMP association is near background under basal (lane 3) and stimulated (lane 4) conditions. Input shows equal CTMP and Akt in all groups, and higher p-Akt in stimulated cells expressing wild-type Akt. n = 3 independent experiments. (f) Ischemia (but not preconditioning) dephosphorylates and activates (destabilizes) PTEN in CA1. n = 5–7 animals per group and time point. Error bars represent means ± SEM. Significance is as described in the legend to Fig. 1.
Figure 4
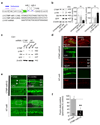
CTMP is critical to ischemia-induced neuronal death. (a) CTMP miRNA-1 (directed to bps 144–164 of CTMP) and CTMP miRNA-2 (directed to bps 453–473 of CTMP) were chained in the lentiviral vector; nontargeting (nontargeting)-miRNA. (b) miRNA was transfected into N2A cells with lipofectamine, for 3–5 days. Left, Westerns of CTMP miRNA-1/2-transfected, nontargeting-miRNA-transfected or untransfected N2A cells were probed for CTMP, p-Akt (Ser 473), Akt, GFP and β-actin. Right, summary of Western data on left. (c) miRNA was unilaterally transduced directly into the right CA1 of intact rats. Westerns of ipsilateral CA1 of CTMP miRNA-1/2 or nontargeting-miRNA-transduced rats were probed for CTMP, p-Ser473-Akt and GFP. c indicates sample from contralateral hippocampus and i indicates sample from ipsilateral hippocampus. (d) GFP expression and Nissl staining of brain sections at the level of the dorsal hippocampus from rats unilaterally transduced in the right hippocampus with CTMP miRNA-1/2 (upper) or nontargeting-miRNA (lower). (e) Fluoro-Jade (FJ) staining of adjacent sections from rats unilaterally transduced in the right hippocampus with CTMP miRNA-1/2 (upper) or nontargeting-miRNA (lower). Arrow shows a needle track. (f) Summary of data in (e); n = 9–10 animals per group. Errors represent means ± SEM. **P < 0.01.
Similar articles
- Carboxy-terminal modulator protein induces Akt phosphorylation and activation, thereby enhancing antiapoptotic, glycogen synthetic, and glucose uptake pathways.
Ono H, Sakoda H, Fujishiro M, Anai M, Kushiyama A, Fukushima Y, Katagiri H, Ogihara T, Oka Y, Kamata H, Horike N, Uchijima Y, Kurihara H, Asano T. Ono H, et al. Am J Physiol Cell Physiol. 2007 Nov;293(5):C1576-85. doi: 10.1152/ajpcell.00570.2006. Epub 2007 Jul 5. Am J Physiol Cell Physiol. 2007. PMID: 17615157 - Activating Transcription Factor 3 Diminishes Ischemic Cerebral Infarct and Behavioral Deficit by Downregulating Carboxyl-Terminal Modulator Protein.
Kao MH, Huang CY, Cheung WM, Yan YT, Chen JJ, Ho YS, Hsu CY, Lin TN. Kao MH, et al. Int J Mol Sci. 2023 Jan 24;24(3):2306. doi: 10.3390/ijms24032306. Int J Mol Sci. 2023. PMID: 36768628 Free PMC article. - Elevated Expression of Carboxy-Terminal Modulator Protein (CTMP) Aggravates Brain Ischemic Injury in Diabetic db/db Mice.
Chen Y, Cai M, Deng J, Tian L, Wang S, Tong L, Dong H, Xiong L. Chen Y, et al. Neurochem Res. 2016 Sep;41(9):2179-89. doi: 10.1007/s11064-016-1932-y. Epub 2016 May 9. Neurochem Res. 2016. PMID: 27161366 - Age-Related Upregulation of Carboxyl Terminal Modulator Protein Contributes to the Decreased Brain Ischemic Tolerance in Older Rats.
Li J, Shan W, Zuo Z. Li J, et al. Mol Neurobiol. 2018 Jul;55(7):6145-6154. doi: 10.1007/s12035-017-0826-6. Epub 2017 Dec 18. Mol Neurobiol. 2018. PMID: 29250714 Free PMC article. - Transcriptional regulation of neuronal genes and its effect on neural functions: expression and function of forkhead transcription factors in neurons.
Fukunaga K, Ishigami T, Kawano T. Fukunaga K, et al. J Pharmacol Sci. 2005 Jul;98(3):205-11. doi: 10.1254/jphs.fmj05001x3. Epub 2005 Jul 9. J Pharmacol Sci. 2005. PMID: 16006742 Review.
Cited by
- Overview of carboxyl‑terminal modulator protein 1 and its importance in various metabolic regulations (Review).
Nguyen H, Kim SH, Juang U, Gwon S, Jung W, Huang Q, Lee S, Lee B, Kwon SH, Park J. Nguyen H, et al. Mol Med Rep. 2024 Sep;30(3):158. doi: 10.3892/mmr.2024.13282. Epub 2024 Jul 12. Mol Med Rep. 2024. PMID: 38994770 Free PMC article. Review. - Pathways Involved in Oxygen Glucose Deprivation Damage of Astrocytes.
Wei S, Tong J, Xue Q, Liu Y, Xu X. Wei S, et al. J Mol Neurosci. 2017 Jan;61(1):115-122. doi: 10.1007/s12031-016-0832-6. Epub 2016 Sep 6. J Mol Neurosci. 2017. PMID: 27601172 - Heat shock protein 70-mediated sensitization of cells to apoptosis by Carboxyl-Terminal Modulator Protein.
Piao L, Li Y, Yang KJ, Park KA, Byun HS, Won M, Hong J, Kim JL, Kweon GR, Hur GM, Seok JH, Cho JY, Chun T, Hess D, Sack R, Maira SM, Brazil DP, Hemmings BA, Park J. Piao L, et al. BMC Cell Biol. 2009 Jul 15;10:53. doi: 10.1186/1471-2121-10-53. BMC Cell Biol. 2009. PMID: 19604401 Free PMC article. - The insulin/IGF signaling regulators cytohesin/GRP-1 and PIP5K/PPK-1 modulate susceptibility to excitotoxicity in C. elegans.
Tehrani N, Del Rosario J, Dominguez M, Kalb R, Mano I. Tehrani N, et al. PLoS One. 2014 Nov 25;9(11):e113060. doi: 10.1371/journal.pone.0113060. eCollection 2014. PLoS One. 2014. PMID: 25422944 Free PMC article. - Novel link of anti-apoptotic ATF3 with pro-apoptotic CTMP in the ischemic brain.
Huang CY, Chen JJ, Wu JS, Tsai HD, Lin H, Yan YT, Hsu CY, Ho YS, Lin TN. Huang CY, et al. Mol Neurobiol. 2015 Apr;51(2):543-57. doi: 10.1007/s12035-014-8710-0. Epub 2014 Apr 26. Mol Neurobiol. 2015. PMID: 24771044
References
- Graham SH, Chen J. Programmed cell death in cerebral ischemia. J. Cereb. Blood Flow Metab. 2001;21:99–109. - PubMed
- Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat. Rev. Neurosci. 2003;4:399–415. - PubMed
- Liou AK, Clark RS, Henshall DC, Yin XM, Chen J. To die or not to die for neurons in ischemia, traumatic brain injury and epilepsy: a review on the stress-activated signaling pathways and apoptotic pathways. Prog. Neurobiol. 2003;69:103–142. - PubMed
- Zukin RS, et al. Molecular and Cellular Mechanisms of Ischemia-Induced Neuronal Death in. In: Mohr JP, Choi DW, Grotta JC, Weir B, Wolf PA, editors. Stroke: Pathophysiology, Diagnosis, and Management. Philadelphia: Churchill Livingstone; 2004. pp. 829–854.
- Ouyang YB, et al. Survival- and death-promoting events after transient cerebral ischemia: phosphorylation of Akt, release of cytochrome C and Activation of caspase-like proteases. J. Cereb. Blood Flow Metab. 1999;19:1126–1135. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials