The L6 protein TM4SF1 is critical for endothelial cell function and tumor angiogenesis - PubMed (original) (raw)
The L6 protein TM4SF1 is critical for endothelial cell function and tumor angiogenesis
Shou-Ching Shih et al. Cancer Res. 2009.
Abstract
Transmembrane-4-L-six-family-1 (TM4SF1) was originally described as a cancer cell protein. Here, we show that it is highly expressed in the vascular endothelium of human cancers and in a banded pattern in the filopodia of cultured endothelial cells (EC). TM4SF1 knockdown prevented filopodia formation, inhibited cell mobility, blocked cytokinesis, and rendered EC senescent. Integrin-alpha5 and integrin-beta1 subunits gave a similar staining pattern and interacted constitutively with TM4SF1, whereas integrin subunits often associated with angiogenesis (alphaV, beta3, beta5) interacted with TM4SF1 only after vascular endothelial growth factor (VEGF)-A or thrombin stimulation. TM4SF1 knockdown substantially inhibited maturation of VEGF-A(164)-induced angiogenesis. Thus, TM4SF1 is a key regulator of EC function in vitro and of pathologic angiogenesis in vivo and is potentially an attractive target for antiangiogenesis therapy.
Figures
Figure 1
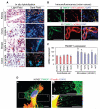
TM4SF1 expression in the vascular endothelium of human cancers and in cultured EC. A, in situ hybridization shows strong TM4SF1 expression in the blood vessels of colon, kidney, ovary, and breast carcinomas. Images shown are representative of those taken from four different patients with each type of cancer. Bright field reveals histology and dark field shows mRNA expression. Arrows, TM4SF1-positive blood vessels. B, immunofluorescence staining of a typical colon cancer with antibodies against TM4SF1 (green) and either (i) VE-cadherin or (ii) anti-human α-smooth muscle actin (red). C, TM4SF1 expression in several types of cultured EC (left) and in HUVEC stimulated with indicated growth factors (right). *, P < 0.05. D, TM4SF1 (green) and F-actin (red) double-staining of HUVEC reveals that TM4SF1 is localized to the plasma membrane, perinuclear vesicles (arrow), and particularly to filopodia (a). Filopodia exhibited a banded staining pattern (b and c). Arrows, filopodia branching (c). Scale bars, 100 μm (A and B) and 10 μm (D).
Figure 2
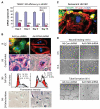
TM4SF1 KD rendered HUVEC senescent. A, TM4SF1 KD efficiency at various times after transfection with 50-moi Ad-hTM4-shRNA or Ad-Con-shRNA. B, day 3 TM4SF1-KD HUVEC lose TM4SF1 staining and develop stress fibers (i), lose filopodia (i and ii), increase cells in G2-M by 4.3× (iii), and express β-galactosidase activity (iv). C, loss of TM4SF1 staining in a spontaneously senescent HUVEC. D, 3 d TM4SF1-KD HUVEC migrate poorly in a wound healing assay, fail to form tubes on Matrigel.
Figure 3
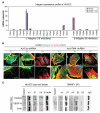
TM4SF1-integrin expression and interactions. A, integrin expression profile in HUVEC ± TM4SF1 KD. B, immunofluorescence staining of integrin α5, αV, and β1 subunits in HUVEC 3 d after 50-moi transfection with either Ad-hTM4-shRNA or Ad-Con-shRNA. Scale bars, 10 μm. C, control HUVEC or HUVEC stimulated for 6 h with VEGF-A (20 ng/mL) or thrombin (THR; 1.5 u/mL). Cell lysates, prepared before and after immunoprecipitation (IP) with anti-TM4SF1 antibody, were immunoblotted with indicated antibodies. IgG and β-actin were loading controls. The anti-TM4SF1 antibody effectively immunoprecipitated native TM4SF1 but reacted poorly with denatured TM4SF1. Expression of β3 and β5 were below detection levels in whole cell lysates.
Figure 4
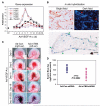
Expression of TM4SF1 and integrin subunits in Ad-VEGF-A164–induced angiogenesis; effect of TM4SF1 on angiogenesis. A, mRNA expression levels of TM4SF1 and integrin subunits in mouse ears at indicated times after injection of 2.5 × 107 plaque-forming unit Ad-VEGF-A164. B, in situ hybridization localization of TM4SF1 mRNA to VM in day 11 Ad-VEGF-A164–injected mouse ears (top). Hematoxylin-stained VM illustrating smooth muscle cell coat (green arrows, bottom). C, angiogenic response in mouse ears at indicated times after Ad-VEGF-A164 injection. Moi (109) of Ad-mTM4-shRNA or Ad-Con-shRNA were injected into the right and left ears, respectively, 1 d before Ad-VEGF-A164 injection. The experiment was repeated four times. D, quantification of angiogenesis with the Evan's Blue dye 19 d after Ad-VEGF-A164 injection.
Similar articles
- Lost miR-141 and upregulated TM4SF1 expressions associate with poor prognosis of pancreatic cancer: regulation of EMT and angiogenesis by miR-141 and TM4SF1 via AKT.
Xu D, Yang F, Wu K, Xu X, Zeng K, An Y, Xu F, Xun J, Lv X, Zhang X, Yang X, Xu L. Xu D, et al. Cancer Biol Ther. 2020 Apr 2;21(4):354-363. doi: 10.1080/15384047.2019.1702401. Epub 2020 Jan 7. Cancer Biol Ther. 2020. PMID: 31906774 Free PMC article. - TM4SF1: a tetraspanin-like protein necessary for nanopodia formation and endothelial cell migration.
Zukauskas A, Merley A, Li D, Ang LH, Sciuto TE, Salman S, Dvorak AM, Dvorak HF, Jaminet SC. Zukauskas A, et al. Angiogenesis. 2011 Sep;14(3):345-54. doi: 10.1007/s10456-011-9218-0. Epub 2011 May 29. Angiogenesis. 2011. PMID: 21626280 Free PMC article. - Novel Anti-TM4SF1 Antibody-Drug Conjugates with Activity against Tumor Cells and Tumor Vasculature.
Visintin A, Knowlton K, Tyminski E, Lin CI, Zheng X, Marquette K, Jain S, Tchistiakova L, Li D, O'Donnell CJ, Maderna A, Cao X, Dunn R, Snyder WB, Abraham AK, Leal M, Shetty S, Barry A, Zawel L, Coyle AJ, Dvorak HF, Jaminet SC. Visintin A, et al. Mol Cancer Ther. 2015 Aug;14(8):1868-76. doi: 10.1158/1535-7163.MCT-15-0188. Epub 2015 Jun 18. Mol Cancer Ther. 2015. PMID: 26089370 - Neuropilins in the Context of Tumor Vasculature.
Niland S, Eble JA. Niland S, et al. Int J Mol Sci. 2019 Feb 1;20(3):639. doi: 10.3390/ijms20030639. Int J Mol Sci. 2019. PMID: 30717262 Free PMC article. Review. - Reciprocal Dynamics of Metabolism and mRNA Translation in Tumor Angiogenesis.
Lidonnici J, Oberkersch RE. Lidonnici J, et al. Int J Mol Sci. 2024 Oct 20;25(20):11284. doi: 10.3390/ijms252011284. Int J Mol Sci. 2024. PMID: 39457064 Free PMC article. Review.
Cited by
- TM4SF19 controls GABP-dependent YAP transcription in head and neck cancer under oxidative stress conditions.
Shin E, Kwon Y, Jung E, Kim YJ, Kim C, Hong S, Kim J. Shin E, et al. Proc Natl Acad Sci U S A. 2024 Feb 13;121(7):e2314346121. doi: 10.1073/pnas.2314346121. Epub 2024 Feb 5. Proc Natl Acad Sci U S A. 2024. PMID: 38315837 Free PMC article. - Heterogeneity of the tumor vasculature.
Nagy JA, Chang SH, Shih SC, Dvorak AM, Dvorak HF. Nagy JA, et al. Semin Thromb Hemost. 2010 Apr;36(3):321-31. doi: 10.1055/s-0030-1253454. Epub 2010 May 20. Semin Thromb Hemost. 2010. PMID: 20490982 Free PMC article. Review. - Clinical significance of TM4SF1 as a tumor suppressor gene in gastric cancer.
Peng XC, Zeng Z, Huang YN, Deng YC, Fu GH. Peng XC, et al. Cancer Med. 2018 Jun;7(6):2592-2600. doi: 10.1002/cam4.1494. Epub 2018 Apr 17. Cancer Med. 2018. PMID: 29665316 Free PMC article. - Deciphering the composition and key driver genes of breast invasive micropapillary carcinoma by multi-omics analysis.
Xie Y, Liu Z, Zhang J, Li G, Ni B, Shi C, Zou Y, Zhou Y, Shang X. Xie Y, et al. iScience. 2024 Oct 16;27(11):111178. doi: 10.1016/j.isci.2024.111178. eCollection 2024 Nov 15. iScience. 2024. PMID: 39524324 Free PMC article. - Intracellular distribution of TM4SF1 and internalization of TM4SF1-antibody complex in vascular endothelial cells.
Sciuto TE, Merley A, Lin CI, Richardson D, Liu Y, Li D, Dvorak AM, Dvorak HF, Jaminet SC. Sciuto TE, et al. Biochem Biophys Res Commun. 2015 Sep 25;465(3):338-43. doi: 10.1016/j.bbrc.2015.07.142. Epub 2015 Aug 1. Biochem Biophys Res Commun. 2015. PMID: 26241677 Free PMC article.
References
- Hellstrom I, Horn D, Linsley P, et al. Monoclonal mouse antibodies raised against human lung carcinoma. Cancer Res. 1986;46:3917–23. - PubMed
- O'Donnell RT, DeNardo SJ, Shi XB, et al. L6 monoclonal antibody binds prostate cancer. Prostate. 1998;37:91–7. - PubMed
- DeNardo SJ, O'Grady LF, Macey DJ, et al. Quantitative imaging of mouse l-6 monoclonal antibody in breast cancer patients to develop a therapeutic strategy. Int J Rad Appl Instrum B. 1991;18:621–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 CA92644/CA/NCI NIH HHS/United States
- P01 CA092644-06A1/CA/NCI NIH HHS/United States
- S10 RR017927-01A2/RR/NCRR NIH HHS/United States
- P01 CA092644/CA/NCI NIH HHS/United States
- S10 RR017927/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources