Disruption of FADS2 gene in mice impairs male reproduction and causes dermal and intestinal ulceration - PubMed (original) (raw)
. 2009 Sep;50(9):1870-80.
doi: 10.1194/jlr.M900039-JLR200. Epub 2009 Apr 7.
Takayuki Y Nara, Manuel Roqueta-Rivera, Emily C Radlowski, Peter Lawrence, Ying Zhang, Byung H Cho, Mariangela Segre, Rex A Hess, J Thomas Brenna, Wanda M Haschek, Manabu T Nakamura
Affiliations
- PMID: 19351970
- PMCID: PMC2724775
- DOI: 10.1194/jlr.M900039-JLR200
Disruption of FADS2 gene in mice impairs male reproduction and causes dermal and intestinal ulceration
Chad K Stroud et al. J Lipid Res. 2009 Sep.
Abstract
Delta-6 desaturase (D6D) catalyzes the first step in the synthesis of highly unsaturated fatty acids (HUFA) such as arachidonic (AA), docosapentaenoic (DPAn-6), and docosahexaenoic (DHA) acids, as well as the last desaturation of DPAn-6 and DHA. We created D6D-null mice (-/-), which enabled us to study HUFA deficiency without depleting their precursors. In -/-, no in vivo AA synthesis was detected after administration of [U-(13)C]linoleic acid (LA), indicating absence of D6D isozyme. Unexpectedly, all of the -/- developed ulcerative dermatitis when fed a purified diet lacking D6D products but containing ample LA. The -/- also exhibited splenomegaly and ulceration in duodenum and ileocecal junction. Male -/- lacked normal spermatozoa with a severe impairment of spermiogenesis. Tissue HUFAs in -/- declined differentially: liver AA and DHA by 95%, and a smaller decrease in brain and testes. Dietary AA completely prevented dermatitis and intestinal ulcers in -/-. DPAn-6 was absent in -/- brain under AA supplementation, indicating absence of D6D isozyme for DPAn-6 synthesis from AA. This study demonstrated a distinct advantage of the D6D-null mice (-/-) to elucidate (1) AA function without complication of LA deprivation and (2) DHA function in the nervous system without AA depletion or DPAn-6 replacement seen in traditional models.
Figures
Fig. 1.
Synthetic pathway of highly unsaturated fatty acids (HUFA) (A); design of the targeting vector (B); and selection of ES cells by PCR (C) and by Southern blot (D). A: In mammals, HUFA (AA, DPA n-6 and DHA) are synthesized from LA and ALA. B: The vector consists of neomycin resistant cassette (Neo), 6.1 kb and 1 kb flanking arms homologous to wild-type, and diphtheria toxin gene (DTA). Neo replaces a promoter and exon 1 region of FADS2. C: PCR screening of neomycin resistant ES cell lines. PCR with P1 and P3 primers (upper panel) shows presence of wild-type sequence in all cell lines, whereas PCR with P2 and P3 primers (lower panel) shows homologous recombination only in lanes 1, 2, 4 and 6. D: Southern blot analysis of EcoRV-digested DNA from PCR positive ES cells. Probe 3 (upper panel) shows a positive band of 4.1 kb on lanes 1, 6, and 10. Probe 5 (middle panel) also shows a positive band of 12.2 kb on lanes 1, 6, and 10. Neo Probe (bottom panel) shows positive bands of right size (4.1 kb) on lanes 1, 5, 6, and 10, whereas lanes 4, 5, and 7 show bands with different sizes indicating random incorporation of targeting vector.
Fig. 2.
Determination of absence of D6D isozyme. A: Gene expression measured by SYBR Green demonstrates halted transcription of FADS2, whereas activity of desaturating 14C-LA was present in liver microsomes of the knockout. The double bond position could not be determined by this activity assay. Values are the mean ± SD (n = 3); * P < 0.05; Student s _t_-test. B: Separation of liver phospholipid fatty acids by gas chromatography. 18:3 n-6 and 20:3 n-6 peaks were absent in the −/− (dashed). A peak that was unique to −/− near 20:3 n-6 was identified as Δ7,11,14-20:3. C: Output of covalent adduct chemical ionization GC/MS that identified the presence of 7,11,14-20:3 in liver phospholipids. D: Summary of desaturation, elongation pathway for the fatty acids detected in the liver from −/−. E: Transcripts of putative fatty acid desaturases in liver: FADS3, fatty acid desaturase 3; FADS6, fatty acid desaturase 6; RIKEN, 4833423E24Rik. Values are the mean ± SD (n = 3); * P < 0.05 by Student s _t_-test. F: In vivo 13C labeling of liver fatty acids 48 h after administration of [U-13C]LA. Values of individual animals (n = 4 each) are shown. D6D desaturation products 18:3 n-6, 20:3 n-6, and 20:4 n-6 were absent in all −/− animals.
Fig. 3.
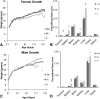
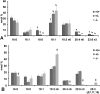
Growth and relative organ weights of +/+, +/− and −/− animals fed AIN93 diet after weaning. A: Growth in the females showed no significant differences. B: Organ weight of females at ∼21 weeks of age. C: Growth in the males. Weight of −/− plateaued after 80 days of age. D: Organ weight of males at ∼20 weeks of age. *P < 0.05; †P < 0.01; ‡P < 0.001 by Student s _t_-test (−/− compared with +/+); n = 3–6.
Fig. 4.
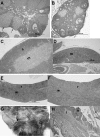
Histology of female mice fed AIN93 diet from weaning. +/+, +/−, and −/− animals were euthanized at the manifestation of dermatitis in −/− (∼21weeks of age). No difference in histology was observed between +/+ and +/−. A: Ovary from +/+ mouse shows normal follicle maturation with multiple corpora lutea (arrows), whereas (B) ovary from −/− mouse is smaller and lacks mature corpora lutea. C: Thymus from +/+ mouse has normal cortex (c) and medulla (m), while (D) thymus from −/− mouse is small with severe cortical lymphocyte depletion. E: Spleen from +/+ mouse has normal white (w) and red (r) pulp. F: Spleen from −/− mouse is greatly enlarged (4.0×) due to expanded red pulp (r) whereas white pulp (w) is depleted. G: Ulcerative dermatitis (arrow) on head of −/− at necropsy. H: Ulceration of −/− skin with superficial exudation (e) and severe inflammation (i) of the dermis. H and E stain.
Fig. 5.
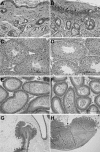
Histology of male mice fed AIN93 diet from weaning. +/+, +/−, and −/− animals were euthanized at the manifestation of dermatitis in −/− (∼20 weeks of age). No difference in histology was observed between +/+ and +/−. Skin from a −/− mouse, (A) unaffected area, and (B) with ulcerative dermatitis (arrows). C: Seminiferous tubules in testis from +/+ mouse shows all stages of spermatogenesis including elongated spermatids (arrows) and spermatozoa (in lumen), while (D) testis from −/− mouse shows lack of elongated spermatids and spermatozoa. E: Epididymis from +/+ mouse contains viable spermatozoa (bracket), while (F) epididymis from −/− mouse contains sloughed round spermatids (smaller cells with round nuclei) and spermatocytes (larger cells with more dispersed chromatin). G: The intestine from a −/− mouse has an ulcer (bracket) at the ileocecal junction. H: Duodenum ulcer (arrow) in −/− with inflammation extending into the submucosa. No intestinal ulceration was found in +/+ and +/− animals. H and E stain.
Fig. 6.
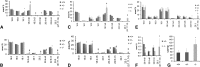
Tissue fatty acid profile and liver triglycerides from ++, +/−, and −/− fed AIN93 diet from weaning. Tissues were analyzed at the onset of dermatitis in the −/− animals. A: Fatty acids in liver phospholipids from females and (C) from males. B: Fatty acids in brain total lipid extracts from females and (D) from males. E: Fatty acids in testis total lipid extracts. F: Fatty acids in cauda epididymis total lipid extracts. G: Liver triglycerides in males. *P < 0.05; †P < 0.01, ‡P < 0.001by Student's _t_-test (−/−compared with +/+); n = 3 (females), 4 (males).
Fig. 7.
Tissue fatty acid profile. A: Female liver cholesterol esters from D6D +/+, +/− and −/−. Fatty acids from +/− fall in between those of +/+ and −/−, indicating that a single copy of the D6D gene is unable to fully support HUFA synthesis. Difference between data with different letters are significant by Tukey s test after ANOVA (P < 0.05). B: Male heart fatty acids from D6D +/+, +/− and −/−. Moderate amounts of 20:3 (7, 11, 14) were accumulated. Student s t_-test (between +/+ and −/−); ‡_P < 0.05.
Fig. 8.
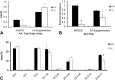
Effects of dietary supplementation of AA. AIN93G diet with or without 0.4% AA were fed to +/+ and −/− females (n = 3 each). Groups without AA were terminated at 4 months of age, whereas AA supplemented groups were terminated at 8 months of age. A: AA levels in total skin lipid extracts. B: PGD2 from skin samples. Values are shown relative to the nonsupplemented +/+ group. C: Fatty acid profile of brain total lipid extracts from AA supplemented animals. *P < 0.05; †P < 0.01; ‡ P < 0.001 by Student's _t_-test.
Similar articles
- Docosahexaenoic acid supplementation fully restores fertility and spermatogenesis in male delta-6 desaturase-null mice.
Roqueta-Rivera M, Stroud CK, Haschek WM, Akare SJ, Segre M, Brush RS, Agbaga MP, Anderson RE, Hess RA, Nakamura MT. Roqueta-Rivera M, et al. J Lipid Res. 2010 Feb;51(2):360-7. doi: 10.1194/jlr.M001180. Epub 2009 Aug 18. J Lipid Res. 2010. PMID: 19690334 Free PMC article. - The metabolism and distribution of docosapentaenoic acid (n-6) in the liver and testis of growing rats.
Tam PS, Sawada R, Cui Y, Matsumoto A, Fujiwara Y. Tam PS, et al. Biosci Biotechnol Biochem. 2008 Oct;72(10):2548-54. doi: 10.1271/bbb.80249. Epub 2008 Oct 7. Biosci Biotechnol Biochem. 2008. PMID: 18838818 - Peroxisome proliferator-activated receptor alpha is required for feedback regulation of highly unsaturated fatty acid synthesis.
Li Y, Nara TY, Nakamura MT. Li Y, et al. J Lipid Res. 2005 Nov;46(11):2432-40. doi: 10.1194/jlr.M500237-JLR200. Epub 2005 Aug 16. J Lipid Res. 2005. PMID: 16106047 - Essential fatty acid synthesis and its regulation in mammals.
Nakamura MT, Nara TY. Nakamura MT, et al. Prostaglandins Leukot Essent Fatty Acids. 2003 Feb;68(2):145-50. doi: 10.1016/s0952-3278(02)00264-8. Prostaglandins Leukot Essent Fatty Acids. 2003. PMID: 12538078 Review. - Metabolism and functions of highly unsaturated fatty acids: an update.
Nakamura MT, Cho HP, Xu J, Tang Z, Clarke SD. Nakamura MT, et al. Lipids. 2001 Sep;36(9):961-4. doi: 10.1007/s11745-001-0806-5. Lipids. 2001. PMID: 11724468 Review.
Cited by
- Omega-3 fatty acid supplementation and cardiovascular disease.
Jump DB, Depner CM, Tripathy S. Jump DB, et al. J Lipid Res. 2012 Dec;53(12):2525-45. doi: 10.1194/jlr.R027904. Epub 2012 Aug 17. J Lipid Res. 2012. PMID: 22904344 Free PMC article. Review. - Health implications of high dietary omega-6 polyunsaturated Fatty acids.
Patterson E, Wall R, Fitzgerald GF, Ross RP, Stanton C. Patterson E, et al. J Nutr Metab. 2012;2012:539426. doi: 10.1155/2012/539426. Epub 2012 Apr 5. J Nutr Metab. 2012. PMID: 22570770 Free PMC article. - Trans fatty acid intake is inversely related to total sperm count in young healthy men.
Chavarro JE, Mínguez-Alarcón L, Mendiola J, Cutillas-Tolín A, López-Espín JJ, Torres-Cantero AM. Chavarro JE, et al. Hum Reprod. 2014 Mar;29(3):429-40. doi: 10.1093/humrep/det464. Epub 2014 Jan 12. Hum Reprod. 2014. PMID: 24419496 Free PMC article. - Juniperonic Acid Biosynthesis is Essential in Caenorhabditis Elegans Lacking Δ6 Desaturase (fat-3) and Generates New ω-3 Endocannabinoids.
Guha S, Calarco S, Gachet MS, Gertsch J. Guha S, et al. Cells. 2020 Sep 19;9(9):2127. doi: 10.3390/cells9092127. Cells. 2020. PMID: 32961767 Free PMC article. - Acyl-CoA synthetase 6 enriches seminiferous tubules with the ω-3 fatty acid docosahexaenoic acid and is required for male fertility in the mouse.
Hale BJ, Fernandez RF, Kim SQ, Diaz VD, Jackson SN, Liu L, Brenna JT, Hermann BP, Geyer CB, Ellis JM. Hale BJ, et al. J Biol Chem. 2019 Sep 27;294(39):14394-14405. doi: 10.1074/jbc.RA119.009972. Epub 2019 Aug 9. J Biol Chem. 2019. PMID: 31399511 Free PMC article.
References
- Burr G. O., Burr M. M. 1930. On the nature and role of the fatty acids essential in nutrition. J. Biol. Chem. 86: 587–621.
- Cho H. P., Nakamura M. T., Clarke S. D. 1999. Cloning, expression, and nutritional regulation of the mammalian delta-6 desaturase. J. Biol. Chem. 274: 471–477. - PubMed
- Williard D. E., Nwankwo J. O., Kaduce T. L., Harmon S. D., Irons M., Moser H. W., Raymond G. V., Spector A. A. 2001. Identification of a fatty acid delta6-desaturase deficiency in human skin fibroblasts. J. Lipid Res. 42: 501–508. - PubMed
- Hansen H. S., Jensen B. 1985. Essential function of linoleic acid esterified in acylglucosylceramide and acylceramide in maintaining the epidermal water permeability barrier. Evidence from feeding studies with oleate, linoleate, arachidonate, columbinate and alpha-linolenate. Biochim. Biophys. Acta. 834: 357–363. - PubMed
- Hartop P. J., Prottey C. 1976. Changes in transepidermal water loss and the composition of epidermal lecithin after applications of pure fatty acid triglycerides to the skin of essential fatty acid-deficient rats. Br. J. Dermatol. 95: 255–264. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases