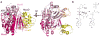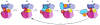Ubiquitin-like protein activation by E1 enzymes: the apex for downstream signalling pathways - PubMed (original) (raw)
Review
Ubiquitin-like protein activation by E1 enzymes: the apex for downstream signalling pathways
Brenda A Schulman et al. Nat Rev Mol Cell Biol. 2009 May.
Abstract
Attachment of ubiquitin or ubiquitin-like proteins (known as UBLs) to their targets through multienzyme cascades is a central mechanism to modulate protein functions. This process is initiated by a family of mechanistically and structurally related E1 (or activating) enzymes. These activate UBLs through carboxy-terminal adenylation and thiol transfer, and coordinate the use of UBLs in specific downstream pathways by charging cognate E2 (or conjugating) enzymes, which then interact with the downstream ubiquitylation machinery to coordinate the modification of the target. A broad understanding of how E1 enzymes activate UBLs and how they selectively coordinate UBLs with downstream function has come from enzymatic, structural and genetic studies.
Figures
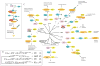
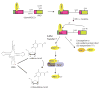
Figure 1. Diverse functions of Ubiquitin-like proteins (UBLs)
a | The canonical conjugation pathway for ubiquitin (Ub). The E1 enzyme ubiquitin activating enzyme 1 (UBA1) reacts with ubiquitin to form a ubiquitin-adenylate intermediate. Ubiquitin is transferred to a Cys in the catalytic domain of UBA1 to form the activated ubiquitin~UBA1 complex. A second molecule of ubiquitin binds to the adenylation domain and is converted to ubiquitin-adenylate. The doubly loaded E1 complex is then recognized by a cognate E2 ubiquitin conjugating enzyme, which receives ubiquitin to form a ubiquitin-charged E2. E2s recognize E3s that are associated with substrates and transfer ubiquitin to the substrate. Multiple cycles of binding to charged E2s leads to the formation of ubiquitin chains, which are recognized by the 26S Proteasome, facilitating substrate degradation. b | Enzymatic mechanism of the ubiquitin activation and conjugation cycle. E1 – UBA1; Ub(A) – ubiquitin associated noncovalently at the adenylation active site; Ub(T) – ubiquitin covalently linked to the catalytic Cys of UBA1 through a thioester bond. c |The pathways that employ ubiquitin-like proteins (UBLs) (yellow) are arranged around the phylogenetic tree for the 17 UBLs that are known to be conjugated to other molecules via their carboxyl-terminal Gly residue. E1 proteins (purple) for specific UBLs can be monomeric (UBA1, UBA6, UBE1L), heterodimeric (UBA2/SAE1, UBA3/APPBP1), or homodimeric (UBA4, ATG7, and likely UBA5). E2 proteins (green) associate with E1 proteins and receive the activated UBL via a trans-thioesterification reaction. E2s then transfer their UBLs to substrates (orange), typically via association is an E3 ubiquitin ligase.
Figure 2. Activation of a prokaryotic ubiquitin-like protein, MoaD, by MoeB
a | Overall structure of the Escherichia coli molybdopterin biosynthetic enzyme B (MoeB) (magenta, pink)-MoaD (yellow, orange)-ATP homodimer (1JWA.PDB), shown in two orientations rotated by 70° around the x-axis. The left view highlights the MoaD carboxyl-terminus approaching ATP for adenylation, and the right view highlights the dimeric structure of an adenylation domain. b | Schematic view of MoeB-catalyzed adenylation of MoaD, highlighting the roles of the Mg2+-coordinating Asp of MoeB and the MoeB’ Arg finger.
Figure 3. E1 domain structures and enzymatic mechanism
a | Primary structures of human canonical and noncanoncal E1 enzymes, with domains indicated and coloured according to the legend (bottom), and adenylation domains aligned according to molybdopterin biosynthetic enzyme B (MoeB) and thiamine biosynthesis enzyme F (ThiF) primary structures shown above. Lines reflect insertions in sequences between conserved domains. b | Cartoon view of canonical E1 crystal structures with ubiquitin (yellow) associated noncovalently at the adenylation active site of Saccharomyces cerevisiae Uba1 (3CMM.PDB), c | with small ubiquitin-related modifier (SUMO)- 1 (yellow) and ATP associated noncovalently at the adenylation active site of SAE1-UBA2 (1Y8R.PDB), and d | with NEDD8 (yellow) and ATP noncovalently at the adenylation active site of NAE1-UBA3 (1R4M.PDB). The domains are coloured according to the schematic view in panel a, and oriented as the left view of MoeB in FIG. 2a.
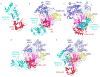
Figure 4. Canonical E1 domain rotation in ubiquitin-like protein transfer to E2s
a | E1–E2 interactions shown by the crystal structure of the isolated ubiquitin-fold domain (UFD) from the UBA3 subunit of the E1 of NEDD8 (red) complexed with the core domain from UBC12 (cyan), the E2 of NEDD8 (1Y8X.PDB). The sulfhydryl of the catalytic Cys in Ubc12 is shown as a green sphere. b–d, Models of E2s (cyan) bound to structures of E1-UBL(A) complexes, with arrows highlighting E1-to-E2 Cys-to-Cys distances, for the E1 of SUMO (1Y8R.PDB) and Ubc9 (1U9B.PDB) (b), the yeast ubiquitin E1 (3CMM.PDB) and Ubc2 (2AYZ.PDB) (c), and the E1 of NEDD8 (1R4M.PDB) and Ubc12 (1Y8X.PDB) (d). e | UFD rotation revealed from the structure of the doubly UBL-loaded/E2-bound NEDD8 E1 [NAE1-UBA3~NEDD8(T)-NEDD8(A)-MgATPUbc12(catalytically inactive mutant] (2NVU.PDB). NEDD8(T) is in orange, NEDD8(A) is in yellow, and the location that would correspond to a Ubc12 catalytic Cys is shown as a green sphere. E1-UBL structures are oriented and coloured as in FIG. 3, and E1 and E2 catalytic Cys residues are shown in green.
Figure 5. Model for a thioester switch modulating E1–E2 interactions
a | E1 conformation as for the NAE1-UBA3-NEDD8(A) complex, modelled with the carboxyl terminus of the adenylation active site (U(A)) covalently linked to AMP. b | After double-UBL-loading of the E1, the thioester-bound UBL would clash with the ubiquitin-fold domain (UFD) of E1 in the position observed in the NAE1 -UBA3-NEDD8(A) structure. Thus, double-UBL-loading might restrict positions accessible by the thioester active site of UBL (UBL(T)) and the UFD of E1. c | With the rotation of the E1’s UFD observed in the NAE1-UBA3~NEDD8(T)-NEDD8(A)-MgATP-Ubc12(catalytically inactive mutant) structure, two cryptic E2-binding sites are unmasked, facilitating the doubly-UBLloaded E1 binding to or positioning E2 for the UBL transfer reaction. d | Following UBL transfer to the catalytic Cys of E2, the covalent tether of UBL(T) to E1 is eliminated. e | Steric clashing between E1 and E2~UBL might further facilitate the release of this product, and reset the E1 for another activation cycle. The first UBL to enter the cascade is coloured in orange, and the second in yellow.
Figure 6. The UBA4/MOCS3 pathway
Uba4 and its mammalian homologue MOCS3 function to promote both sulfur transfer reactions through URM1 as well as the conjugation of URM1 to other proteins via an isopeptide bond. URM1 forms an adenylate intermediate with the adenylation domain of MOCS3 or Uba4. A reactive Cys (residue 397) in the Rhodanese homology domain (RHD) of UBA4 becomes persulfurated likely via the action of nitrogen fixing bacteria S-like protein (Nfs1) in a reaction that requires Cysteine (Cys). Through a process that is poorly understood from a mechanistic perspective, the adenylate on URM1 is replaced by a sulfur from the persulfide on Cys397, forming URM1 thiocarboxylate. URM1-thiocarboxylate associates with the ATP binding subunit of a heterodimeric enzyme complex required for conversion of uridine-mcm5 in the anti-codon of U-rich tRNAs to the 2-thiouridine-mcm5 derivative. In yeast, this complex is composed of Needs CLA4 for Survival 2 (Ncs2), Ncs6, and a previously unstudied protein Yor251c. The ATP associated with Ncs6 has been proposed to make an adenylate with uridine, as an intermediate for the trans-thiolation reaction. 2-Thiouridine-mcm5 is crucial for the fidelity of translation. In a separate process, URM1 has been proposed to be conjugated to the alkyl hydroperoxide reductase Ahp1 in yeast but the molecular intermediates in this process are not defined.
Similar articles
- Dual E1 activation systems for ubiquitin differentially regulate E2 enzyme charging.
Jin J, Li X, Gygi SP, Harper JW. Jin J, et al. Nature. 2007 Jun 28;447(7148):1135-8. doi: 10.1038/nature05902. Nature. 2007. PMID: 17597759 - Basis for a ubiquitin-like protein thioester switch toggling E1-E2 affinity.
Huang DT, Hunt HW, Zhuang M, Ohi MD, Holton JM, Schulman BA. Huang DT, et al. Nature. 2007 Jan 25;445(7126):394-8. doi: 10.1038/nature05490. Epub 2007 Jan 14. Nature. 2007. PMID: 17220875 Free PMC article. - The basis for selective E1-E2 interactions in the ISG15 conjugation system.
Durfee LA, Kelley ML, Huibregtse JM. Durfee LA, et al. J Biol Chem. 2008 Aug 29;283(35):23895-902. doi: 10.1074/jbc.M804069200. Epub 2008 Jun 26. J Biol Chem. 2008. PMID: 18583345 Free PMC article. - E1 Enzymes as Therapeutic Targets in Cancer.
Barghout SH, Schimmer AD. Barghout SH, et al. Pharmacol Rev. 2021 Jan;73(1):1-58. doi: 10.1124/pharmrev.120.000053. Pharmacol Rev. 2021. PMID: 33177128 Review. - Chemical Tools for Probing the Ub/Ubl Conjugation Cascades.
Kochańczyk T, Fishman M, Lima CD. Kochańczyk T, et al. Chembiochem. 2025 Jan 2;26(1):e202400659. doi: 10.1002/cbic.202400659. Epub 2024 Nov 6. Chembiochem. 2025. PMID: 39313481 Free PMC article. Review.
Cited by
- E2/E3-independent ubiquitin-like protein conjugation by Urm1 is directly coupled to cysteine persulfidation.
Ravichandran KE, Kaduhr L, Skupien-Rabian B, Shvetsova E, Sokołowski M, Krutyhołowa R, Kwasna D, Brachmann C, Lin S, Guzman Perez S, Wilk P, Kösters M, Grudnik P, Jankowska U, Leidel SA, Schaffrath R, Glatt S. Ravichandran KE, et al. EMBO J. 2022 Oct 17;41(20):e111318. doi: 10.15252/embj.2022111318. Epub 2022 Sep 14. EMBO J. 2022. PMID: 36102610 Free PMC article. - SUMOylation targeting mitophagy in cardiovascular diseases.
Xiao H, Zhou H, Zeng G, Mao Z, Zeng J, Gao A. Xiao H, et al. J Mol Med (Berl). 2022 Nov;100(11):1511-1538. doi: 10.1007/s00109-022-02258-4. Epub 2022 Sep 26. J Mol Med (Berl). 2022. PMID: 36163375 Review. - Uncovering DUB Selectivity through an Ion Mobility-Based Assessment of Ubiquitin Chain Isomers.
Shestoperova EI, Strieter ER. Shestoperova EI, et al. Anal Chem. 2023 Nov 28;95(47):17416-17423. doi: 10.1021/acs.analchem.3c04622. Epub 2023 Nov 14. Anal Chem. 2023. PMID: 37962301 Free PMC article. - Ubiquitination and the Ubiquitin-Proteasome System as regulators of transcription and transcription factors in epithelial mesenchymal transition of cancer.
Voutsadakis IA. Voutsadakis IA. Tumour Biol. 2012 Aug;33(4):897-910. doi: 10.1007/s13277-012-0355-x. Epub 2012 Mar 6. Tumour Biol. 2012. PMID: 22399444 Review. - A substrate-trapping strategy to find E3 ubiquitin ligase substrates identifies Parkin and TRIM28 targets.
Watanabe M, Saeki Y, Takahashi H, Ohtake F, Yoshida Y, Kasuga Y, Kondo T, Yaguchi H, Suzuki M, Ishida H, Tanaka K, Hatakeyama S. Watanabe M, et al. Commun Biol. 2020 Oct 20;3(1):592. doi: 10.1038/s42003-020-01328-y. Commun Biol. 2020. PMID: 33082525 Free PMC article.
References
- Ciechanover A. Intracellular protein degradation: from a vague idea, through the lysosome and the ubiquitin-proteasome system, and onto human diseases and drug targeting (Nobel lecture) Angew Chem Int Ed Engl. 2005;44:5944–5967. - PubMed
- Goldberg AL. Functions of the proteasome: from protein degradation and immune surveillance to cancer therapy. Biochem Soc Trans. 2007;35:12–17. - PubMed
- Hershko A, Heller H, Elias S, Ciechanover A. Components of ubiquitin-protein ligase system. Resolution, affinity purification, and role in protein breakdown. J Biol Chem. 1983;258:8206–8214. Describes the chromatographic separation of E1, E2 and E3 activities and demonstrates reconstitution of the first ubiquitin ligase reaction. - PubMed
- Hochstrasser M. Lingering mysteries of ubiquitin-chain assembly. Cell. 2006;124:27–34. - PubMed
- Pickart CM, Fushman D. Polyubiquitin chains: polymeric protein signals. CurrOpin Chem Biol. 2004;8:610–616. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM077053/GM/NIGMS NIH HHS/United States
- R01 GM069530/GM/NIGMS NIH HHS/United States
- R01 GM070565/GM/NIGMS NIH HHS/United States
- R03 AG022633/AG/NIA NIH HHS/United States
- R01 GM054137/GM/NIGMS NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R01 AG011085/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous