Sweet taste receptor expressed in pancreatic beta-cells activates the calcium and cyclic AMP signaling systems and stimulates insulin secretion - PubMed (original) (raw)
Sweet taste receptor expressed in pancreatic beta-cells activates the calcium and cyclic AMP signaling systems and stimulates insulin secretion
Yuko Nakagawa et al. PLoS One. 2009.
Abstract
Background: Sweet taste receptor is expressed in the taste buds and enteroendocrine cells acting as a sugar sensor. We investigated the expression and function of the sweet taste receptor in MIN6 cells and mouse islets.
Methodology/principal findings: The expression of the sweet taste receptor was determined by RT-PCR and immunohistochemistry. Changes in cytoplasmic Ca(2+) ([Ca(2+)](c)) and cAMP ([cAMP](c)) were monitored in MIN6 cells using fura-2 and Epac1-camps. Activation of protein kinase C was monitored by measuring translocation of MARCKS-GFP. Insulin was measured by radioimmunoassay. mRNA for T1R2, T1R3, and gustducin was expressed in MIN6 cells. In these cells, artificial sweeteners such as sucralose, succharin, and acesulfame-K increased insulin secretion and augmented secretion induced by glucose. Sucralose increased biphasic increase in [Ca(2+)](c). The second sustained phase was blocked by removal of extracellular calcium and addition of nifedipine. An inhibitor of inositol(1, 4, 5)-trisphophate receptor, 2-aminoethoxydiphenyl borate, blocked both phases of [Ca(2+)](c) response. The effect of sucralose on [Ca(2+)](c) was inhibited by gurmarin, an inhibitor of the sweet taste receptor, but not affected by a G(q) inhibitor. Sucralose also induced sustained elevation of [cAMP](c), which was only partially inhibited by removal of extracellular calcium and nifedipine. Finally, mouse islets expressed T1R2 and T1R3, and artificial sweeteners stimulated insulin secretion.
Conclusions: Sweet taste receptor is expressed in beta-cells, and activation of this receptor induces insulin secretion by Ca(2+) and cAMP-dependent mechanisms.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
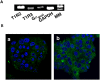
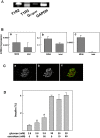
Figure 1. Expression of the sweet taste receptor in MIN6 cells.
(A) Expression of mRNA for T1R2, T1R3 and α subunit of gustducin (Gαgust) in MIN6 cells was measured by RT-PCR. MM: molecular markers. The result is a representative of three experiments. (B) MIN6 cells were stained by anti-T1R2 (a) and anti-T1R3 (b) antibodies.
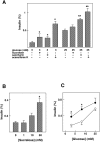
Figure 2. Effect of artificial sweeteners on insulin secretion in MIN6 cells.
(A) MIN6 cells were incubated for 60 min with or without 50 mM saccharin, 50 mM sucralose or 50 mM acesulfame-K in the presence of 3 or 25 mM glucose, and insulin secretion was measured. Values are expressed as means±S.E. for four experiments. *: P<0.05 vs 3 mM glucose, **: P<0.05 vs 25 mM glucose. Note that 50 mM mannitol did not affect insulin secretion. (B) MIN6 cells were incubated for 60 min with 0, 1, 10 or 50 mM sucralose in the presence of 3 mM glucose, and insulin secretion was measured. Values are expressed as mean±S. E. for four experiments. *: P<0.05 vs 3 mM glucose alone. (C) MIN6 cells were incubated for 60 min with 3, 8.3 or 25 mM glucose in the presence (•) and absence (○) of 50 mM sucralose. Values are expressed as mean±S. E. for four experiments. *: P<0.05 vs without sucralose.
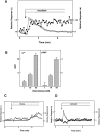
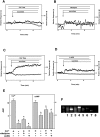
Figure 3. Effect of sucralose on [Ca2+]c and [cAMP]c in MIN6 cells.
(A) MIN6 cells expressing Epac1-camps were loaded with fura-2, and changes in [Ca2+]c (○) and [cAMP]c (•) were monitored. Cells were stimulated by 50 mM sucralose. Note that 50 mM mannitol did not affect [Ca2+]c or [cAMP]c indicating that the effect of sucralose was not simply due to changes in osmolarity. (B) Dose-response relationship for the effect of sucralose. Cells were stimulated with various concentrations of sucralose, and the area under the curve (AUC) for [Ca2+]c and [cAMP]c was calculated. Values are the mean±S.E. for five experiments. (C) Epac1-camps-expressing cells loaded with fura-2 were stimulated with 25 mM glucose, and changes in [Ca2+]c (○) and [cAMP]c (•) were monitored. (D) Cells were stimulated with 50 µM carbachol and changes in [Ca2+]c (○) and [cAMP]c (•) were monitored.
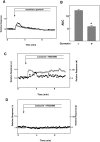
Figure 4. Involvement of the sweet taste receptor in the action of sucralose.
(A): Epac1-camps-expressing MIN6 cells loaded with fura-2 were incubated with (○) or without (•) 3 µg/ml gurmarin for 10 min and then stimulated with 50 mM sucralose. Changes in [Ca2+]c were monitored. (B) Quantitative analysis of the effect of gurmarin. Cells were stimulated by 50 mM sucralose in the presence and absence of gurmarin and the AUC was calculated. Values are the mean±S.E. for four experiments. *: p<0.05 vs none. (C) Epac1-camps-expressing MIN6 cells loaded with fura-2 were preincubated with 10 µM YM254890 for 10 min and then stimulated with 50 mM sucralose in the presence of YM254890. Changes in [Ca2+]c (○) and [cAMP]c (•) were monitored. (D) Epac1-camps-expressing MIN6 cells loaded with fura-2 were preincubated with YM254890 for 10 min and then stimulated with 50 µM carbachol in the presence (○) and absence (•) of YM254890.
Figure 5. Role of calcium entry in the action of sucralose on [Ca2+]c.
(A) Epac1-camps-expressing MIN6 cells loaded with fura-2 were stimulated by 50 mM sucralose in calcium-free HBSS, and changes in [Ca2+]c (○) and [cAMP]c (•) were monitored. (B) Epac1-camps-expressing MIN6 cells loaded with fura-2 were stimulated by 50 mM sucralose in the presence of 1 µM nifedipine. (C) Epac1-camps-expressing MIN6 cells loaded with fura-2 were incubated in Na-free HBSS and stimulated with 50 mM sucralose and changes in [Ca2+]c (○) and [cAMP]c (•) were measured. (D) Epac1-camps-expressing MIN6 cells loaded with fura-2 were stimulated with 50 mM sucralose in the presence of 200 µM 2APB and changes in [Ca2+]c (○) and [cAMP]c (•) were measured. (E) Quantitative analysis of the above data. *: P<0.05. (F) Expression of TRPM channels in MIN6 cells. Expression of various types of TRPM channels was measured by RT-PCR using mRNA obtained from MIN6 cells. 1, 2, 3, 4, 5, 6, 7, and 8 stand for TRPM1, TRPM2, TRPM3, TRPM4, TRPM5, TRPM6, TRPM7, TRPM8, respectively.
Figure 6. Effect of sucralose on PKC activation.
(A) MIN6 cells expressing MARCKS-GFP were loaded with fura-2. Cells were stimulated with 50 mM sucralose and changes in [Ca2+]c (○) and the amount of MARCKS-GFP in cytosol (•) were monitored. (B) MIN6 cells expressing MARCKS-GFP were loaded with fura-2. Cells were then incubated in Ca2+-free HBSS and stimulated with 50 mM sucralose and changes in [Ca2+]c (○) and amount of MARCKS-GFP in cytosol (•) were measured.
Figure 7. Expression of the sweet receptor in islets.
(A) Expression of mRNA in Mouse Islets. mRNA was extracted from mouse islets and the expression of T1R2, T1R3, and Gαgust was measured by RT-PCR. (B) Comparison of the Expression of T1Rs and Gustducin in Islets and MIN6 cells. mRNA levels for T1R2 (a), T1R3 (b) and Gαgust (c) were measured by quantitative PCR in islets and MIN6 cells and expressed as relative to β actin. (C) Expression of T1R3 in Islets. Pancreatic slices were stained with anti-T1R3 (a) and anti-insulin (b) antibodies. c: merge. (D) Effects of Artificial Sweeteners on Insulin secretion from Islets. Islets were incubated for 60 min with various concentrations of sucralose in the presence of 2.8 and 20 mM glucose. Values are the mean±S.E. for four experiments. *: p<0.05 vs control.
Similar articles
- Glucose-Sensing Receptor T1R3: A New Signaling Receptor Activated by Glucose in Pancreatic β-Cells.
Kojima I, Nakagawa Y, Hamano K, Medina J, Li L, Nagasawa M. Kojima I, et al. Biol Pharm Bull. 2015;38(5):674-9. doi: 10.1248/bpb.b14-00895. Biol Pharm Bull. 2015. PMID: 25947913 - Lactisole inhibits the glucose-sensing receptor T1R3 expressed in mouse pancreatic β-cells.
Hamano K, Nakagawa Y, Ohtsu Y, Li L, Medina J, Tanaka Y, Masuda K, Komatsu M, Kojima I. Hamano K, et al. J Endocrinol. 2015 Jul;226(1):57-66. doi: 10.1530/JOE-15-0102. Epub 2015 May 20. J Endocrinol. 2015. PMID: 25994004 - Multimodal function of the sweet taste receptor expressed in pancreatic β-cells: generation of diverse patterns of intracellular signals by sweet agonists.
Nakagawa Y, Nagasawa M, Mogami H, Lohse M, Ninomiya Y, Kojima I. Nakagawa Y, et al. Endocr J. 2013;60(10):1191-206. doi: 10.1507/endocrj.ej13-0282. Epub 2013 Aug 9. Endocr J. 2013. PMID: 23933592 - Sweet Taste-Sensing Receptors Expressed in Pancreatic β-Cells: Sweet Molecules Act as Biased Agonists.
Kojima I, Nakagawa Y, Ohtsu Y, Medina A, Nagasawa M. Kojima I, et al. Endocrinol Metab (Seoul). 2014 Mar;29(1):12-9. doi: 10.3803/EnM.2014.29.1.12. Endocrinol Metab (Seoul). 2014. PMID: 24741449 Free PMC article. Review. - Role of the glucose-sensing receptor in insulin secretion.
Kojima I, Medina J, Nakagawa Y. Kojima I, et al. Diabetes Obes Metab. 2017 Sep;19 Suppl 1:54-62. doi: 10.1111/dom.13013. Diabetes Obes Metab. 2017. PMID: 28880472 Review.
Cited by
- Artificially Sweetened Beverage Consumption and Cancer Risk: A Comprehensive Dose-Response Meta-Analysis of Prospective Studies.
Yin T, Li J, Wang Y, Liu K, Long T, Cheng L. Yin T, et al. Nutrients. 2022 Oct 22;14(21):4445. doi: 10.3390/nu14214445. Nutrients. 2022. PMID: 36364707 Free PMC article. Review. - The Role of the Sweet Taste Receptor in Enteroendocrine Cells and Pancreatic β-Cells.
Kojima I, Nakagawa Y. Kojima I, et al. Diabetes Metab J. 2011 Oct;35(5):451-7. doi: 10.4093/dmj.2011.35.5.451. Epub 2011 Oct 31. Diabetes Metab J. 2011. PMID: 22111035 Free PMC article. - Unveiling the profound influence of sucralose on metabolism and its role in shaping obesity trends.
Singh S A, Singh S, Begum RF, Vijayan S, Vellapandian C. Singh S A, et al. Front Nutr. 2024 Jul 2;11:1387646. doi: 10.3389/fnut.2024.1387646. eCollection 2024. Front Nutr. 2024. PMID: 39015535 Free PMC article. Review. - Taste Receptors beyond Taste Buds.
Ki SY, Jeong YT. Ki SY, et al. Int J Mol Sci. 2022 Aug 26;23(17):9677. doi: 10.3390/ijms23179677. Int J Mol Sci. 2022. PMID: 36077074 Free PMC article. Review. - Enhanced expression of the sweet taste receptors and alpha-gustducin in reactive astrocytes of the rat hippocampus following ischemic injury.
Shin YJ, Park JH, Choi JS, Chun MH, Moon YW, Lee MY. Shin YJ, et al. Neurochem Res. 2010 Oct;35(10):1628-34. doi: 10.1007/s11064-010-0223-2. Epub 2010 Jul 2. Neurochem Res. 2010. PMID: 20596769
References
- Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJP, et al. Mammalian sweet taste receptors. Cell. 2001;106:381–390. - PubMed
- Zhao GQ, Zhang Y, Hoon MA, Chadrashekar J, Erlenbach I, et al. The receptors for mammalian sweet and umani taste. Cell. 2003;115:255–266. - PubMed
- Nie y, Vigues S, Hobbs JR, Conn GL, Munger SD. Distinct contributions of T1R2 and T1R3 taste receptor subunits to the detection of sweet stimuli. Curr Biol. 2006;15:1948–1952. - PubMed
- Linderman B. Receptors and transduction in taste. Nature. 2002;413:219–225. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous